4263
Acoustically transparent and low-profile head coil for high precision magnetic resonance guided focused ultrasound at 3 T1Weill Cornell Medicine, New York, NY, United States, 2MR Engineering, GE Healthcare, Aurora, OH, United States, 3Department of Neurological Surgery, Weill Cornell Medicine, New York, NY, United States
Synopsis
Magnetic resonance guided focused ultrasound (MRgFUS) is a non-invasive therapeutic modality for neurodegenerative diseases that allows real-time imaging of the targeted regions. However, MR image quality is poor and severely limits the technology due to the use of the body coil for focal targeting. Acoustic simulations demonstrated an acoustic transparency (signal loss <1%) of the coil when used for thalamic sonication. In vivo results showed increase of the SNR over the body coil by a factor of 7.3 and 7.6 in a brain image with and without the presence of the transducer, respectively.
Introduction
Magnetic Resonance guided Focused Ultrasound (MRgFUS) is a non-invasive therapeutic modality for neurodegenerative disorders such as essential tremor and Alzheimer’s Disease that has achieved dramatic success.1-5 However, the MR image quality is extremely poor and severely limits the technology due to the use of the body coil for targeting. This coil is versatile but yields both a very poor signal, and the dark band in MR images typically seen when used with the water-filled metallic transducer placed around the human skull.6 Recent approaches to mitigate this poor image performance issue have included manipulation of the electrical properties of the water by use of doped water7 or dielectric pads.8 Moreover, different head coil have been designed in order to achieve better SNR during focused ultrasound (FUS) treatment. Almost all these methods require costly modification of the INSIGHTEC MRgFUS system, while having associated drawbacks especially regarding their impacts on the acoustic beam. A large hindrance to the success of specific coil designs for MRgFUS has been their acoustic footprint and thus the distortion of ultrasound signal, which ultimately results in a physical shift, signal loss, and/or broadening of the focal point. In this work, we present acoustic simulations and first in vivo MR images with a novel 8-channel head coil design for MRgFUS. The proposed RF coil array is comprised of ultra-thin wiring mounted on acoustically transparent fabric.Methods
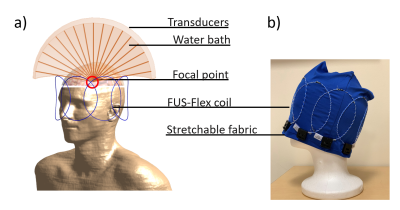
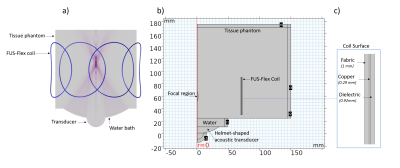
We used a highly flexible receive coil architecture inspired by Air™ Coil technology9-12 to build an 8-channel head coil (FUS-Flex) (Figure 1). This coil is very thin (around 1 mm-diameter) and ultra-flexible, which allows for simple usage during an MRgFUS procedure without any costly modification, as it can be placed directly on the surface of the head.Acoustic footprint: In order to quantify the impact of the coil on the acoustic field, simulations were performed using COMSOL Multiphysics. Figure 2 shows a model of a transducer, water bath, and tissue phantom (radius 150 mm, length 160 mm).13 A coil element was placed at distances of 20 and 80 mm (the latter corresponding to the typical distance of the thalamus) from the focal point as shown in Figure 2b. The transducer is driven at typical low and high frequencies used in FUS treatment, 220 kHz, 650 kHz, and 1 MHz.
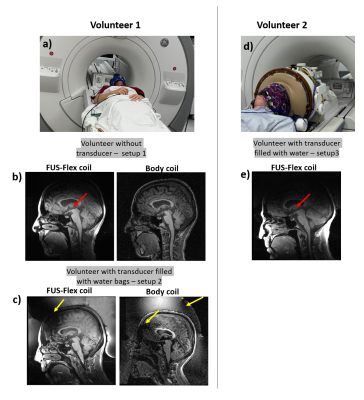
In vivo imaging: First in vivo MR images with the FUS-Flex receive coil were acquired on two healthy volunteers (Figure 4) with and without the transducer and were compared with the body coil in receive mode. In a first test, water-filled bags were placed around the head of a volunteer, while the second volunteer was imaged using the INSIGHTEC sealant membrane and the water-filled transducer helmet. A 3D Bravo sequence (TE = 3 ms, TR = 7.4 ms, FA = 12° and pixel bandwidth= 244.1 Hz/px) was used.
Results
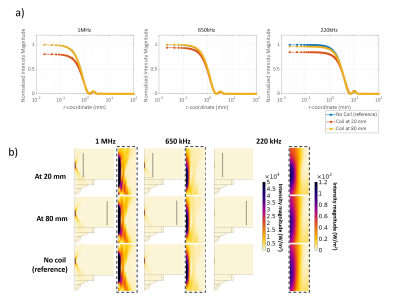
Acoustic simulations: The radial cross-section of the acoustic field magnitude is shown in Figure 3a; results were normalized to the case without a coil. For all frequencies and at a position of the coil at 80 mm, negligible displacement of the focal point or signal loss was found compared to the reference simulation without a coil present (Figure 3b). When the coil was located 20 mm away from the focal point, the focal point shifted by 10 mm and 5 mm at 650 kHz and 220 kHz, respectively. At 1 MHz and 20 mm, no visual shift of the focal point was found. Signal losses at a 20 mm distance were 20%, 5% and 15% for 1 MHz, 650 kHz and 220 kHz, respectively.In vivo imaging: Images acquired using the FUS-Flex coil in Figure 4 depict the position of the thalamus in a healthy volunteer with high sensitivity and show clear improvement of the black band. At the location of the thalamus, the SNR gain is 7.3-fold and 7.6-fold compared to the body coil, with and without the MRgFUS transducer present, respectively.
Discussion and conclusions
The acoustic simulations showed feasibility for the transparency of the coil when it is placed at 80 mm (<1%) from the focal point. For a closer position of the coil to the focal point, the signal loss is between 5 and 20% depending on the emitted frequency. However, most current applications involve deeper regions. Moreover, it should be noted that the attenuation due to the skull is around 70%14, which is much higher than the attenuation due the coil or the related electronics. We showed that the proposed FUS-Flex coil improves the SNR in MRgFUS procedures in presence of helmet-shaped water filled (7.3×) at the thalamus region with an acquisition that is 2 times faster than what can be achieved with the body coil. These results confirm the potential of our proposed FUS-Flex coil, and even higher SNR gains and scan times are expected with a higher channel count. Moreover, the proposed coil is expected to exhibit improved SNR even in conventional, non-MRgFUS, exams.Acknowledgements
This work was supported by the National Institutes of Health (NIH K99/R00 4R00EB024341-03) and GE Healthcare.References
1. Levi Chazen J, Stradford T, Kaplitt MG. Cranial MR-guided Focused Ultrasound for Essential Tremor. Clinical Neuroradiology. 2019;29(2):351-357.
2. Rezai AR, Ranjan M, D’Haese P-F, et al. Noninvasive hippocampal blood−brain barrier opening in Alzheimer’s disease with focused ultrasound. Proceedings of the National Academy of Sciences. 2020;117(17):9180-9182.
3. Fishman P, Lipsman N. Focused ultrasound as an evolving therapy for Parkinson's disease. Movement Disorders. 2019;34(9):1241-1242.
4. Lipsman N, Meng Y, Bethune AJ, et al. Blood–brain barrier opening in Alzheimer’s disease using MR-guided focused ultrasound. Nat Commun. 2018;9(1).
5. Lipsman N, Schwartz ML, Huang Y, et al. MR-guided focused ultrasound thalamotomy for essential tremor: a proof-of-concept study. The Lancet Neurology. 2013;12(5):462-468.
6. Yan X, Allen S, Grissom WA. “Propeller Beanie” Passive Antennas to Alleviate Dark Bands in Transcranial MR-Guided Focused Ultrasound. Proc. Intl. Soc. Mag. Reson. Med. 28; 2020.
7. Collins CM, Brown R, Sodickson DK. Improvement of SNR in MRgFUS with Strategic Design of Bath Medium and Transducer Ground Plane. Proc. Intl. Soc. Mag. Reson. Med. 28; 2020.
8. Pauly KB, Watkins R, Bitton R, et al. RF Shimming in an MRgFUS Brain Transducer with a High Permittivity Material. Proc. Intl. Soc. Mag. Reson. Med. 22; 2014; Milan, Italy.
9. Stack C, Grafendorfer T, Robb F, Falk S, Inventors; General Electric Co, assignee. Flexible radio frequency coil array with detachable straps for mr imaging. US patent US20190154775A1. 2019/05/23/, 2019.
10. Stormont RS, Lindsay SA, Taracila V, et al., Inventors; General Electric Co, assignee. Systems for a radio frequency coil for mr imaging. US patent US20190277926A1. 2019/09/12/, 2019.
11. Stickle Y-J, Follante C, Giancola M, et al. A Novel Ultra-Flexible High-Resolution AIR (Adaptive imaging receive) 64-Channel Bilateral Phased Array for 3T Brachial Plexus MRI. Paper presented at: Proc. Intl. Soc. Mag. Reson. Med2020.
12. Gruber B, Rehner R, Laistler E, Zink S. Anatomically Adaptive Coils for MRI—A 6-Channel Array for Knee Imaging at 1.5 Tesla. Frontiers in Physics. 2020;8.
13. Clavet T. Using Simulation to Study Ultrasound Focusing for Clinical Applications. In. COMSOL Multiphysics2017.
14. Pinton G, Aubry J-F, Bossy E, Muller M, Pernot M, Tanter M. Attenuation, scattering, and absorption of ultrasound in the skull bone. Medical Physics. 2011;39(1):299-307.
Figures