4262
A compact and clonable ultrasound-based sensor system to monitor physiological motion1Radiology, Brigham and Women's Hospital, Harvard Medical School, Boston, MA, United States, 2Computer Science and Engineering, National Sun Yat-sen University, Kaohsiung, Taiwan, 3Amazon Robotics, Westborough, MA, United States
Synopsis
Physiological motion continues to be a problem in MRI and PET, as it can cause blurring and other types of artifacts. We developed a compact, clonable ultrasound-based sensor system that monitors internal motion directly. Alternative means of motion monitoring often involve hardware (including the scanner itself) that is attached to floors, walls and/or ceilings and thus cannot follow the patient from one room to another, from one procedure to the next. The present sensors attach to the skin, they can follow the patient, and as such they may help link different procedures in ways that take internal motion into account.
Introduction
Especially for slower imaging modalities such as MRI or PET, where scan time may reach several minutes, physiological motion is problematic [1-3]. We developed a compact, clonable ultrasound-based sensor system that monitors physiological motion, see Fig. 1-2. This system can be viewed as an alternative to devices such as respiratory bellows [4], spirometers [5] or optical trackers [6-8], as well as algorithms/strategies such as MRI navigator echoes [9] and self-gating in MRI or PET [10, 11]. Potential advantages are considered below.Bellows and optical trackers only detect breathing motion indirectly, through changes on the outline of the torso, and the links between such changes and internal motion are complex [12-17]. Optical trackers require an open line of sight, which can be difficult to achieve in closed-bore systems. Bellows, spirometers and navigator echo implementations typically model respiration as a one-dimensional problem, an oversimplification. The need to gather enough data to generate rudimentary images tends to limit temporal resolution in self-gated methods. Maybe more importantly, navigator echoes, self-gated methods and optical tracking involve hardware (e.g., the scanner itself) that is attached to floors, walls and/or ceilings, and that cannot follow the patient from one room to another, from one procedure to the next.
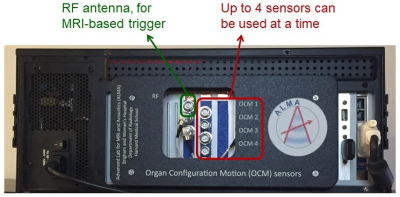
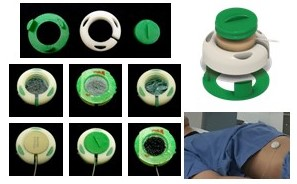
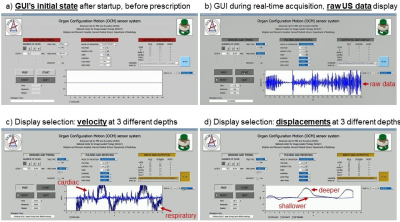
We developed ultrasound-based sensors that attach to the skin (Fig. 2). A clonable system the size of a PC was developed (Fig. 1), controlled though a graphical user interface (GUI) (Fig. 3a). This clonable system is still a one-of-a-kind system, in the sense that it has not been cloned yet, but its existence opens the door to collaborations across institutions and geographical locations.
Methods and results
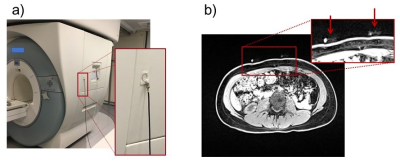
The new system fits within the casing of a PC and handles up to four sensors at a time (red arrow, Fig. 1). An RF antenna, for synchronization with MRI (Fig. 4a), can also be connected (green arrow, Fig. 1). For PET imaging, pulses are sent to the scanner’s respiratory port at pseudo-random intervals, creating timed entries in the raw list-mode data allowing PET and sensor data to be brought to a common time axis for reconstruction.The choice of sensor placement on the thorax is not critical, however, some care should be taken not to place a sensor onto a rib. In general, using two sensors, one on the abdomen just below the ribs and one on the chest, allows respiration to be captured in much of its complexity. The sensors are visible on MRI (Fig. 4b), and as such a record of chosen placement remains preserved in the archived images. To detect cardiac signals, a location closest to the heart would be preferred, although pulsatility is often observed in abdominal signals as well. To place a sensor one simply peels off a protective layer, applies the adhesive surface to the skin, and twists the lid.
As shown in Fig. 3a, the system is operated through a GUI; the ‘sensors and timing’ section controls firings in general terms (number of sensors, triggering, acquisition time), ‘pulsing and receiving’ describes individual firings in more detail (voltage pulses, gain/filters applied to returning signals), while ‘outputs and display’ controls the GUI’s display window, and more generally which data get sent where. Figure 3b-c show screen shots for a one-sensor acquisition, with raw data (b), velocity (c) or displacements (d) being displayed. While raw traces get overwritten in real-time, velocity and displacement values are shown using a scrolling display instead (Fig. 3c-d). Three depths are displayed: shallow (0-3.3cm, pale blue), intermediate (3.3-6.6cm), and deepest (6.6-10cm, darkest blue). Shallow depths correspond to small velocity and displacement because sensors are attached to the skin and mostly move with it. Inspiration/expiration is associated with positive/negative velocities in Fig. 3c, and pulsatility due to cardiac activity is also seen. The number of reconstructed depth intervals, Ndepth, can be modified; with Nocm the number of sensors, the system generates Nocm×Ndepth breathing waveforms.
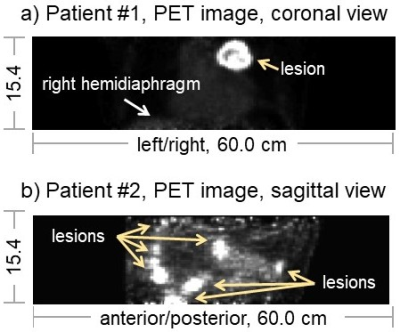
Sensors can be used with MRI or PET; for example, unpublished data from two PET patients are shown in Fig. 5. Patient 1 had a large partly-necrotic lesion in the upper-left lung that was mostly stationary with breathing, while patient 2 was a mesothelioma patient with multiple lesions some of which were highly mobile. Sensor signals were used to reconstruct respiratory gates, and breathing motion would readily be seen for Patient 2 if/when presented in movie format.
Discussion and Conclusion
We believe a key advantage of the present sensors is that they attach to the skin, and as such they can accompany patients through sequential procedures. In principle, procedures could be on different days, in which case sensors would be re-installed at the same marked locations on the skin. The main goal of the present abstract was to communicate the existence of the clonable system (Fig. 1 and 2), which opens possibilities for collaborations that transcend geographical location. Possible applications include linking data from MRI and PET in a manner that takes breathing into account, motion correction in PET, cardiac gating in high-field MRI, or fetal/placenta imaging, for example. We hope that, in time, the present device may take its proper place in the collection of ancillary hardware available to support and enhance modern medical imaging.Acknowledgements
Support from NIH grants R03EB025546 and P41EB015898 is duly acknowledged.References
1. McClelland JR, Hawkes DJ, Schaeffter T, King AP: Respiratory motion models: a review. Med Image Anal 2013;17(1):19-42.
2. Gratz M, Ruhlmann V, Umutlu L, Fenchel M, Hong I, Quick HH: Impact of respiratory motion correction on lesion visibility and quantification in thoracic PET/MR imaging. PLoS One 2020;15(6):e0233209.
3. Petibon Y, Sun T, Han PK, Ma C, El Fakhri G, Ouyang J: MR-based cardiac and respiratory motion correction of PET: application to static and dynamic cardiac 18F-FDG imaging. Phys Med Biol 2019;64(19):195009.
4. Hope TA, Verdin EF, Bergsland EK, Ohliger MA, Corvera CU, Nakakura EK: Correcting for respiratory motion in liver PET/MRI: preliminary evaluation of the utility of bellows and navigated hepatobiliary phase imaging. EJNMMI Phys 2015;2(1):21.
5. Zhang T, Keller H, O'Brien MJ, Mackie TR, Paliwal B: Application of the spirometer in respiratory gated radiotherapy. Med Phys 2003;30(12):3165-71.
6. Zaitsev M, Maclaren J, Herbst M: Motion artifacts in MRI: A complex problem with many partial solutions. J Magn Reson Imaging 2015;42(4):887-901.
7. Nehmeh SA, Erdi YE, Pan T, Pevsner A, Rosenzweig KE, Yorke E, Mageras GS, Schoder H, Vernon P, Squire O, Mostafavi H, Larson SM, Humm JL: Four-dimensional (4D) PET/CT imaging of the thorax. Med Phys 2004;31(12):3179-86.
8. Dawood M, Lang N, Jiang X, Schafers KP: Lung motion correction on respiratory gated 3-D PET/CT images. IEEE Trans Med Imaging 2006;25(4):476-85.
9. Wang Y, Grimm RC, Felmlee JP, Riederer SJ, Ehman RL: Algorithms for extracting motion information from navigator echoes. Magn Reson Med 1996;36(1):117-23.
10. Ren S, Jin X, Chan C, Jian Y, Mulnix T, Liu C, Carson RE: Data-driven event-by-event respiratory motion correction using TOF PET list-mode centroid of distribution. Phys Med Biol 2017;62(12):4741-4755.
11. Schleyer PJ, Thielemans K, Marsden PK: Extracting a respiratory signal from raw dynamic PET data that contain tracer kinetics. Phys Med Biol 2014;59(15):4345-56.
12. Koch N, Liu HH, Starkschall G, Jacobson M, Forster K, Liao Z, Komaki R, Stevens CW: Evaluation of internal lung motion for respiratory-gated radiotherapy using MRI: Part I--correlating internal lung motion with skin fiducial motion. Int J Radiat Oncol Biol Phys 2004;60(5):1459-72.
13. Chi PC, Balter P, Luo D, Mohan R, Pan T: Relation of external surface to internal tumor motion studied with cine CT. Med Phys 2006;33(9):3116-23.
14. Fayad H, Pan T, Pradier O, Visvikis D: Patient specific respiratory motion modeling using a 3D patient's external surface. Med Phys 2012;39(6):3386-95.
15. Dasari P, Johnson K, Dey J, Lindsay C, Shazeeb M, Mukherjee J, Zheng S, King M: MRI Investigation of the Linkage Between Respiratory Motion of the Heart and Markers on Patient’s Abdomen and Chest: Implications for Respiratory Amplitude Binning List-Mode PET and SPECT Studies. IEEE Trans Nucl Sci 2014;61(1):192-201.
16. Ruan D, Fessler JA, Balter JM, Berbeco RI, Nishioka S, Shirato H: Inference of hysteretic respiratory tumor motion from external surrogates: a state augmentation approach. Phys Med Biol 2008;53(11):2923-3.
17. Dasari PK, Shazeeb MS, Konik A, Lindsay C, Mukherjee JM, Johnson KL, King MA: Adaptation of the modified Bouc-Wen model to compensate for hysteresis in respiratory motion for the list-mode binning of cardiac SPECT and PET acquisitions: testing using MRI. Med Phys 2014;41(11):112508.
Figures