4261
Reduced-FOV 3D MR-ARFI with a joint reconstruction for localizing the focused ultrasound beam in neuromodulation1Biomedical Engineering, Vanderbilt University, Nashville, TN, United States, 2Vanderbilt University Institute of Imaging Science, Nashville, TN, United States, 3Radiology and Radiological Sciences, Vanderbilt University Medical Center, Nashville, TN, United States
Synopsis
Magnetic resonance acoustic radiation force imaging (MR-ARFI) can be used to localize the focal spot in MR-guided focused ultrasound (FUS) by encoding the ultrasound-induced displacements into the phase of an MR image. A two-minute reduced-FOV 3D MR-ARFI scan with a joint image reconstruction method at 3 Tesla is proposed to image and localize the entire focus in FUS neuromodulation with a low FUS duty-cycle of 0.85%. In this work the reduced FOV MR-ARFI pulse sequence is demonstrated and the proposed k-space undersampling with joint sparse difference image reconstruction to achieve a two-minute scan time is validated.
Introduction
Magnetic resonance acoustic radiation force imaging (MR-ARFI)1 can confirm the position, size, and strength of a focused ultrasound (FUS) focus using motion-encoding gradients (MEGs) to encode ultrasound-induced displacements into an MR image. For FUS neuromodulation in the brain, MR-ARFI is valuable to image the FUS focus which can shift and become distorted by skull aberrations, but it is necessary to use a very low duty-cycle of FUS bursts with short durations to avoid heating which could lead to adverse bioeffects2,3. In this work, we present a two-minute 3D reduced-FOV spin-echo MR-ARFI sequence with a joint sparse-difference reconstruction using complimentary k-space under-sampling to minimize the scan time with a very low FUS duty-cycle.Methods
Pulse sequence and k-space sampling scheme: Figure 1a illustrates the desired imaging geometry for the sequence, which is a 40×40×160-mm3 rectangular column whose long axis aligns with the ultrasound axial dimension. A 3D reduced-FOV spin-echo MR-ARFI sequence (Figure 2a) with unipolar MEGs was implemented on a 3.0-Tesla human MRI scanner (Philips Ingenia Elition). The in-plane FOV was reduced by moving the refocusing pulse to the in-plane phase-encoded axis (y), enabling the excitation of the rectangular slab which covers the entire focus. The selective RF excitation and refocusing pulses were designed by the Shinnar-Le Roux algorithm4. Root-flipping5 was applied to the refocusing pulse to minimize its peak amplitude. The blipped gradients of the segmented EPI sequence were applied on the through-plane phase-encoded axis (z) to minimize the scan time further. A pair of unipolar MEGs were placed before and after the refocusing pulse. The second MEG was synchronized with a FUS emission. The images with FUS bursts (FUS-ON) and without FUS bursts (FUS-OFF) were alternated between TRs to maintain a low FUS duty-cycle.Figure 2b shows the overview of k-space sampling patterns. A single MR-ARFI scan can obtain four 3D images with switched polarity MEGs and with FUS on and off (XON+, XON-, XOFF+, XOFF-). The positive and negative MEGs were alternated between odd and even kz lines. In other words, the acquired k-space data (YON+, YON-, YOFF+, YOFF-) were under-sampled equidistantly in the kz-direction by an acceleration factor 2. Partial Fourier encoding was applied in the ky-direction. Moreover, the FUS-ON and FUS-OFF k-space data were acquired from the “left half” and “right half” separately, which enables complimentary k-space between images.
Data Acquisition: All data were acquired using a 2-channel phased-array coil (Flex-M; Philips, Healthcare). The following imaging parameters were applied: FOV 160×40×40 mm3, 2×2×2 mm3 isotropic resolution, flip angle 90°, TE/TR 34 ms/500 ms, and EPI factor of 3 lines per TR. The under-sampled data were acquired with partial sampling factors of 0.65 and 1.0. The unipolar MEGs of 40 mT/m and 8 ms were used for ARFI displacement encoding with a trigger delay of -2 ms. Figure 1b shows the FUS experimental setup. A single-element transducer was used for experiments (H115MR, Sonic Concepts, Bothell, WA) to generate sonications at 802kHz with a low duty-cycle (8.5 ms per 1000 ms, free field pressure 2.268 MPa). Sonications were performed on a tissue-mimicking phantom (1% agar/4% graphite weight by volume).
Data reconstruction: The under-sampled k-space data were reconstructed using a joint POCS-based reconstruction method leveraging the sparse complex differences between MR-ARFI images and data consistency as below. F denotes the Fourier transform operator and P is the under-sampling operator.
$$\min_{(X^{ON+},X^{ON-},X^{OFF+},X^{OFF-})}(\parallel X^{ON+}-X^{OFF+}\parallel_{1},\parallel X^{ON+}-X^{OFF-}\parallel_{1},\parallel X^{ON-}-X^{OFF+}\parallel_{1},\parallel X^{ON-}-X^{OFF-}\parallel_{1})$$
Subject to:
$$PFX^{ON+}=X^{ON+}, PFX^{ON-}=X^{ON-}, PFX^{OFF+}=X^{OFF+}, PFX^{OFF-}=X^{OFF-}$$
Displacement maps were calculated by complex phase subtraction of FUS-ON and FUS-OFF images with positive and negative MEGs6 (γ is the gyromagnetic ratio, G is the gradient strength and τ is the gradient duration):
$$\frac{\angle((X^{ON+}\cdot conj(X^{OFF+}))\cdot conj(X^{ON-}\cdot conj(X^{OFF-})))}{2\gamma G\tau}$$
Results
Figure 3a shows three planes of the reduced-FOV MR-ARFI images obtained with a ball phantom and full-FOV images as reference. Figure 3b shows the reduced-FOV images with full k-space sampling and high-resolution full-FOV images of the tissue-mimicking phantom with the FUS experimental setup.Figure 4a shows the fully-sampled ARFI images and the reconstructed FUS-ON ARFI images with positive MEGs.Figure 4b shows the fully-sampled and reconstructed displacement maps with partial sampling factors (p) of 1 and 0.65. The mean displacements (μm) over a 3×3×3-voxel region at the focus were 2.70 (fully-sampled), 2.68 (p=1) and 2.69 (p=0.65). The corresponding scan times were 392 sec, 196 sec, and 132 sec.
Discussion
The results indicated the proposed 3D reduced-FOV MR-ARFI scan can acquire four volumetric ARFI images within about 2 minutes while covering the entire focus effectively. Interleaving the FUS-ON and FUS-OFF image acquisition enables a very low duty-cycle for FUS neuromodulation, while the joint sparse image reconstruction enables the moderate undersampling required for a short scan time, which may need to be repeated many times when finding, steering, or phase-correcting the focus. In future work, the sensitivity and the displacement SNR will be further optimized to improve the proposed protocol.Conclusion
The proposed 3D reduced-FOV MR-ARFI sequence and the joint reconstruction method enables rapid acquisition of a volumetric view of the acoustic beam with a low FUS duty-cycle and while preserving the visibility of the FUS beam focus at 3T. The presented work will enhance the procedure of targeting the focus during FUS neuromodulation.Acknowledgements
This work was supported by NIH grant U18 EB 029351 (HEAL).References
- Nathan McDannold and Stephan E Maier. Magnetic resonance acoustic radiation force imaging. Medical Physics, 35(8):3748-3758, 2008.
- Horder MM, Barnett SB, Vella GJ, Edwards MJ, Wood AK. In vivo heating of the guinea-pig fetal brain by pulsed ultrasound and estimates of thermal index. Ultrasound in medicine & biology. 1998 Dec 1;24(9):1467-74.
- Plaksin M, Kimmel E, Shoham S. Cell-type-selective effects of intramembrane cavitation as a unifying theoretical framework for ultrasonic neuromodulation. eNeuro. 2016 May;3(3).
- Pauly J, Le Roux P, Nishimura D, Macovski A. Parameter relations for the Shinnar-Le Roux selective excitation pulse design algorithm (NMR imaging). IEEE transactions on medical imaging. 1991 Mar;10(1):53-65.
- Sharma A, Lustig M, Grissom WA. Root‐flipped multiband refocusing pulses. Magnetic resonance in medicine. 2016 Jan;75(1):227-37.
- Chen J, Watkins R, Pauly KB. Optimization of encoding gradients for MR‐ARFI. Magnetic Resonance in Medicine. 2010 Apr;63(4):1050-8.
Figures
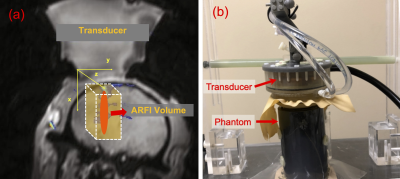
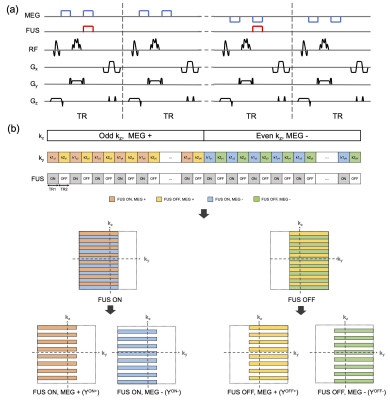
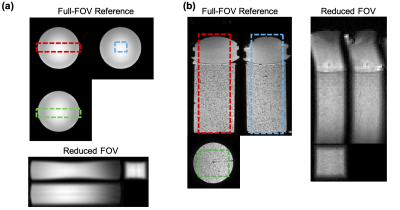
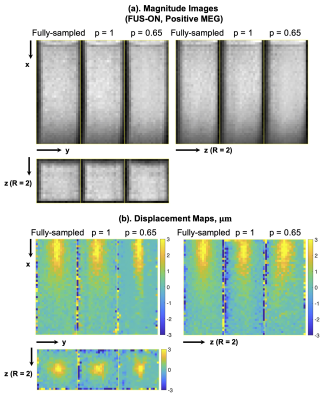
Figure 4. Representative reconstructed magnitude images (FUS-ON, positive MEGs) (a) and corresponding ARF displacement maps (b).