4260
Comparison of Automated Thalamic Segmentation Techniques: Applications in MRgFUS Planning1University of Sydney, Sydney, Australia, 2GE Healthcare, Sydney, Australia, 3St Vincent's Hospital Sydney, Sydney, Australia
Synopsis
Investigation of thalamic segmentation tools FreeSurfer and THOMAS for use in MRgFUS planning.
Introduction
The ventral intermediate nucleus (Vim) is a common target for treatment of tremor with Magnetic Resonance Guided Focused Ultrasound (MRgFUS). MRgFUS uses the targeted delivery of ultrasonic waves to deliver thermal energy to tissue in the brain resulting in the destruction of tissue at the focal point of the ultrasound transducers. The borders of the Vim are not visible on conventional structural imaging, thus improved methods for Vim localisation could aid in treatment planning and patient outcomes. We investigated the thalamic segmentation generated with FreeSurfer1 and the recently developed algorithm, THOMAS2; and explored their potential applications in MRgFUS for essential tremor (ET) and tremor dominant Parkinson’s disease (PD).Methods
Sixty-five subjects (mean age = 72.40 ) with medication-refractory ET and tremor dominant PD were treated with MRgFUS at St Vincent’s Hospital Sydney. Ultrasonic lesioning was performed using the ExAblate Neuro system (InSightec, Tirat Carmel, Israel) 650kHz, with a 1024-element, phased array ultrasound transducer. The initial target for the Vim was chosen by the clinical team using the conventional stereotactic location 25% of the AC-PC distance anterior to the PC, 14mm lateral to the midline at the level of the intercommisural line; and the final target determined with a series of clinically-informed, non-destructive low energy sonication’s. MR imaging was acquired 1-7 days prior to, and immediately after, treatment with MRgFUS. All imaging data was acquired on a 3 Tesla MRI scanner (SIGNA Architect, General Electric, Milwaukee).The pre-treatment and post treatment imaging protocol included a sagittal 3D T1-WI. For 35 of the 65 subjects, the pre-treatment protocol also included an axial WMnMPRAGE3. The MRgFUS ablation site was identified and segmented on the T1-WI acquired immediately after the MRgFUS procedure using ITK-SNAP4. Thalamic parcellation was performed using FreeSurfer (version 6.0) and THOMAS (version 2.1). FreeSurfer was run on the pre-treatment T1-WI in all patients. In a subset of patients, THOMAS was run on the pre-treatment WMnMPRAGE images. The overlap of the ablation site with both FreeSurfer and THOMAS thalamic segmentations for each subject was investigated by multiplying a binary mask of each nucleus with a binary mask of the ablation site and calculating the volume of the overlapping voxels. The THOMAS VLp segmentation (VLPTHOM), which contains the Vim, was investigated by calculation of the overlap with the FreeSurfer thalamic segmentation.Results
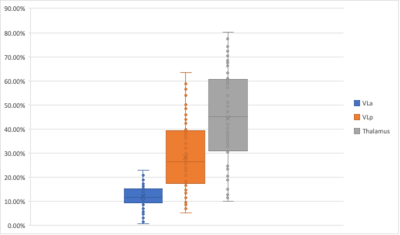
The mean total ablation volume was 136.03 mm3. The mean percentage lesion volume within the FreeSurfer defined thalamus, as a proportion of the total lesion volume, was 44.50%. All subjects had some degree of lesioning within the Ventral lateral anterior (VLaFS) and Ventral lateral posterior (VLpFS) nuclei. The mean percentage lesion volume within the VLaFS and VLpFS as a proportion of the total lesion volume was 11.82% for the VLa and 28.27% respectively (Figure 1). The ablation site overlapped almost exclusively with the VLPTHOM, with a mean of 23.51% of the ablation volume within the VLPTHOMand the remainder outside the THOMAS defined thalamus. There was little overlap of the ablation with any other nuclei segmented by THOMAS when considering the mean of all subjects; only the VLPTHOM had a mean overlap greater than 0.01% of the total ablation volume. VLPTHOM did not overlap particularly well with VLpFS, with a DICE coefficient of 0.54. VLPTHOMwas located further anteriorly, largely corresponding with the combined FreeSurfer VLa and VLp (VLaFS and VLpFS). There was a greater overlap between VLPTHOM and the FreeSurfer VL (which was defined as the combined VLa and VLp), with a DICE coefficient of 0.65. The mean percentage of the total VLPTHOM volume overlapping with VLaFS and VLpFS was 34.08% and 54.12% respectively.Discussion
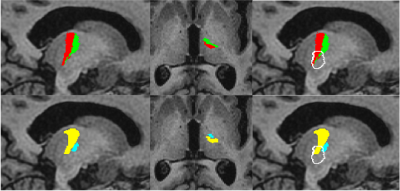
The high degree of overlap between the segmented MRgFUS ablations with the FreeSurfer VLa and VLp segmentations demonstrated that the location of the FreeSurfer ventral lateral nuclei was generally in agreement with the final target chosen during the procedure using the conventional landmark-based technique. The fraction of the total ablation within the thalamus was as low as 10.11%, yet all patients achieved a clinically significant reduction in tremor at the time of treatment. The fact that placement of an ablation only partially within the thalamus can exert a similar clinical effect to an ablation almost entirely within the thalamus suggests that ablation of the Vim itself might not be the most important factor in tremor suppression. The tremor suppression observed with ablations primarily outside of the thalamus, and thus outside of the Vim, might be explained by the fact that the ablation is likely still within the dentato-rubro-thalamic tract (DRT) as it enters the thalamus, which is supported by deep brain stimulation studies5,6,7,8. In the absence of histological correlation, it is not possible to comment on which segmentation was more accurate. However, the location chosen by the clinicians for MRgFUS ablation was generally at the junction of the FreeSurfer VLa and VLp, which agreed with the THOMAS definition of the VLp (Figure 2).Conclusion
In this investigation, we demonstrate the potential application of automated thalamic parcellation to MRgFUS treatment planning for patients with essential tremor and tremor dominant PD. The VLa and VLp nuclei, segmented retrospectively by FreeSurfer and THOMAS, showed substantial agreement with the final ablation target chosen by the treating clinician.Acknowledgements
The authors thank Kirsten Moffat and the radiology team at St Vincent's hospital Sydney for their assistance with data acquisition and logistics.
References
1. J. E. Iglesias et al., “A probabilistic atlas of the human thalamic nuclei combining ex vivo MRI and histology,” Neuroimage, vol. 183, no. July, pp. 314–326, 2018, doi: 10.1016/j.neuroimage.2018.08.012.
2. J. H. Su et al., “Thalamus Optimized Multi Atlas Segmentation (THOMAS): fast, fully automated segmentation of thalamic nuclei from structural MRI,” Neuroimage, vol. 194, no. October 2018, pp. 272–282, 2019, doi: 10.1016/j.neuroimage.2019.03.021.
3. B. Bender, C. Mänz, A. Korn, T. Nägele, and U. Klose, “Optimized 3D magnetization-prepared rapid acquisition of gradient echo: Identification of thalamus substructures at 3T,” Am. J. Neuroradiol., vol. 32, no. 11, pp. 2110–2115, 2011, doi: 10.3174/ajnr.A2705.
4. P. A. Yushkevich et al., “User-guided 3D active contour segmentation of anatomical structures: Significantly improved efficiency and reliability,” NeuroImage (Orlando, Fla.), vol. 31, no. 3, pp. 1116–1128, 2006.
5. V. A. Coenen et al., “The dentato-rubro-thalamic tract as the potential common deep brain stimulation target for tremor of various origin: an observational case series,” Acta Neurochir. (Wien)., 2020, doi: 10.1007/s00701-020-04248-2.
6. A. J. Fenoy and M. C. Schiess, “Deep Brain Stimulation of the Dentato-Rubro-Thalamic Tract: Outcomes of Direct Targeting for Tremor,” Neuromodulation, vol. 20, no. 5, pp. 429–436, 2017, doi: 10.1111/ner.12585
7. J. A. Sweet, B. L. Walter, K. Gunalan, A. Chaturvedi, C. C. McIntyre, and J. P. Miller, “Fiber tractography of the axonal pathways linking the basal ganglia and cerebellum in Parkinson disease: Implications for targeting in deep brain stimulation: Clinical article,” J. Neurosurg., vol. 120, no. 4, pp. 988–996, 2014, doi: 10.3171/2013.12.JNS131537.
8. V. A. Coenen, N. Allert, S. Paus, M. Kronenbürger, H. Urbach, and B. Mädler, “Modulation of the Cerebello-Thalamo-Cortical network in thalamic deep brain stimulation for tremor: A diffusion tensor imaging study,” Neurosurgery, vol. 75, no. 6, pp. 657–669, 2014, doi: 10.1227/NEU.0000000000000540.
Figures