4257
MRI-based tumor localisation after tantalum clip placement for proton beam therapy planning of uveal melanoma1Radiology, Leiden University Medical Center, Leiden, Netherlands, 2Ophthalmology, Leiden University Medical Center, Leiden, Netherlands, 3Radiotherapy, Leiden University Medical Center, Leiden, Netherlands
Synopsis
For proton beam therapy (PBRT) of Uveal Melanoma, tantalum clips are surgically sutured on the outside of the eye to mark the tumour edge. To aid in the 3D clip localisation and modelling of the tumor, a dedicated MRI protocol was developed and evaluated. In 51% of the clips, the MRI and per-operative measurements differed less than 1.0mm. In 28% of the clips the discrepancy between the MRI and peroperative measurement was attributed to the complex tumor geometry or anterior tumor localisation. For the majority of the patients MRI added valuable information to the peroperative measurements, improving PBRT planning.
Introduction
Uveal melanoma (UM) is the most common primary intraocular malignancy in adults, with an incidence of 6 cases per million per year.1,2 Proton beam radiation therapy (PBRT) is the preferred eye sparing treatment for large sized UM or UM located adjacent to the optic nerve.3 Currently, small, 2.5mm diameter,4 tantalum clips (Altomed Limited, Boldon, Tyne and Wear, England) are surgically sutured near the tumour border on the sclera and serve as radiographic markers to mark the tumour edge. During peroperative fundoscopy the tumor casts a shadow on the outer wall of the globe, allowing to measure the clip-tumor distances. These clip-tumor distances form the basis for the geometrical model of the eye used for radiotherapy planning. To aid in the 3D localisation of the clips and the modelling of the tumor for ocular PBRT, a dedicated MRI protocol was developed. Is this study we evaluate these MR-based clip-tumour measurements.Methods
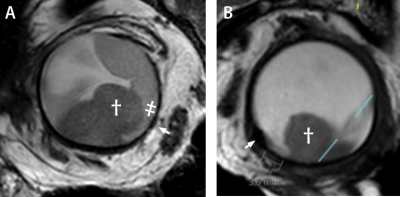
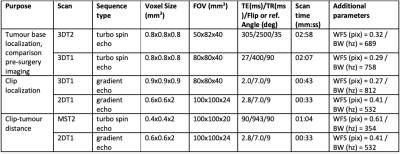
23 patients with the diagnosis of UM were scanned with a dedicated protocol as part of the clinical work-up for PBRT planning on a 3T Philips MRI scanner with a 47mm Rx-surface coil. This dedicated MRI protocol was based on the earlier work of Ferreira et al,5 but modified to limit the susceptibility artefacts of the tantalum clips by localized second order shimming and increasing the gradient strength to at least 45 mT/m. Scan planes were adjusted to optimally depict the geometric relation between the clips and tumour.The protocol consisted of isotropic 3D T1 and T2 sequences, followed by multi-slice T2-weighted sequences with a higher in-plane resolution. A separate set of these 2D scan were acquired for each clip and planned perpendicular to the base of the tumour (figure 1C). To aid clip-detection on T2 TSE, a T1-weighted gradient echo sequence was added. The latter was also helpful to differentiate tumor from associated retinal detachment (figure 1E).
Sequence related differences in size of the clip artefact were evaluated in 3 patients. The clip-tumour distances were measured by a radiologist in all patients and compared with the peroperative measurements of the ophthalmologist. For each clip with more than 1mm difference, the most likely origin of this discrepancy was discussed in a multidisciplinary setting by ophthalmologists, radiologists, radiotherapist and MR-physicist
Results
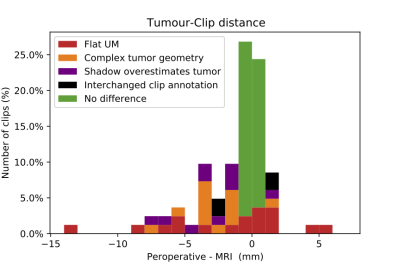
The gradient echo sequences showed significantly larger signal-voids (3DT1: 5.1±0.4 mm, 2DT1: 5.3±0.4 mm) compared to the spin echo sequences (3DT2: 3.0±0.4 mm, 2DT2: 3.0±0.6 mm, p<0.01). The 2DT2 scan, used to determine the clip-tumor distance, showed an overestimation of clip size of approximately 0.5mm.In 51% of the clips, the MRI and per-operative measurements differed less than 1.0mm, figure 2. The largest differences, up to 14 mm, were observed in flat melanoma (15% of the clips), due to an unsharp boundary between tumor and adjacent normal choroid/retina. In 10 clips (12%) the discrepancy between the MRI and peroperative measurement was attributed to the complex tumor geometry, figure 3A. In 13 clips (16%), the anterior localisation of the tumor caused an overprojection of the tumor shadow, resulting in an underestimation of the clip-tumor distance during surgery, figure 3B. For these patients, the MR-imaging was considered to be more reliable. Finally, in two patients, the peroperative annotation of two clips appeared to have been interchanged.
Discussion
The dedicated MRI protocol provided a clear localisation, but serious overestimation of the clips on the gradient-echo sequences, while the spin-echo sequence resulted in a minimal overestimation of the clip of 0.3mm in each direction.Depending on the shape and location of the tumor MRI may add valuable information regarding clip-tumor distance. For flat UM, MR-imaging generally underestimates the tumor dimensions and is therefore less suitable than the conventional peroperative measurements. An exception are unpigmented UM, which are also difficult to visualize peroperatively. For these specific patients, MRI could provide a valuable validation of the peroperative measurements. For UM with a complex shape, the conventional simple 2D measurements could not accurately describe the clip-tumor relation. For anteriorly located UM, part of the peroperative optical measurements were inaccurate as the posterior tumor boundary could not be illuminated. For both of these groups the conventional technique resulted in an overestimation of the tumor size. Including 3D visualisation of the clip-tumor relation with MR-imaging in the workup of these patients could therefore result in a smaller target volume for PBRT, sparing more of the patient’s vision.
Conclusion
MRI adds valuable information to peroperative optical measurements of uveal melanoma and clip distances. In particular in complex shaped or anteriorly located tumors this can help to improve PBRT planning.Acknowledgements
Part of this work is part of the research programme Protons4VISION with project number 14654, which is financed by the Netherlands Organisation for Scientific Research (NWO).References
- Dieckmann K, Georg D, Zehetmayer M, Bogner J, Georgopoulos M, Pötter R. LINAC based stereotactic radiotherapy of uveal melanoma: 4 Years clinical experience. Radiother Oncol 2003. https://doi.org/10.1016/S0167-8140(02)00345-6.
- Weis E, Salopek TG, McKinnon JG, Larocque MP, Temple-Oberle C, Cheng T, et al. Management of uveal melanoma: A consensus-based provincial clinical practice guideline. Curr Oncol 2016. https://doi.org/10.3747/co.23.2859.
- Tarlan B, Kıratlı H. Uveal melanoma: Current trends in diagnosis and management. Turk Oftalmoloiji Derg 2016. https://doi.org/10.4274/tjo.37431.
- Oberacker, Eva, et al. Magnetic resonance safety and compatibility of tantalum markers used in proton beam therapy for intraocular tumors: A 7.0 Tesla study. Magnetic Resonance in Medicine 78.4 (2017): 1533-1546. https://doi.org/10.1002/mrm.26534
- Ferreira, T, Grech Fonk L, Jaarsma-Coes M, van Haren G, Marinkovic M, Beenakker J.-W., MRI of Uveal Melanoma. Cancers 2019, 11, 377. https://doi.org/10.3390/cancers11030377
Figures
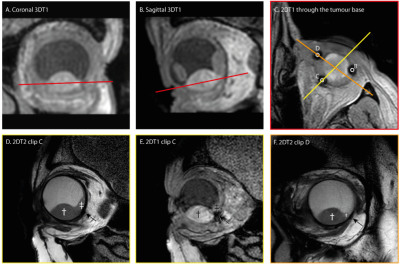
A,B). Coronal and sagittal view of the tumor are used to plan the scan to locate the clips.
C) 2DT1 scan trough the base clearly show the clips and is used to plan the subsequent scans perpendicular to the tumor base and through centre of the tumour and centre of clip C/D.
D,E). T2 spin echo sequence and T1 gradient echo showing the tumour (dagger), retinal detachment (double dagger) and clip (arrow). The clip location is better visualized on the gradient echo sequence, while a more accurate measurement can be made on the spin echo sequence.
F) T2 image of clip D.