4254
Respiratory Motion Detection and Reconstruction Using CAPTURE and Deep Learning for a 0.35T MRI-LINAC System: An Initial Study1Washington University in St. Louis, Saint Louis, MO, United States
Synopsis
Magnetic Resonance Imaging Guided Linear Accelerator (MRI-LINAC) combines an MRI system with a linear accelerator radiotherapy system to treat patients. The MRI-LINAC uses MR to track organ or lesion motion and gates the radiation beam accordingly. In this study, we demonstrate the combination of a T1-weighted self-navigated respiratory motion detection method (CAPTURE) with a deep learning 4D reconstruction (Phase2Phase, P2P) method to derive the 3D deformable respiratory motion field from data acquired on a 0.35 T ViewRay MRI-LINAC system. This initial study demonstrated promising results despite the low SNR at this field strength.
Introduction
An MRI Guided Linear Accelerator (MRI-LINAC) combines MRI with radiotherapy treatment to provide direct visualization of the tumor during treatment. While respiratory and organ motion can have a detrimental effect on treatment delivery, respiratory gating by MRI enables motion mitigation, allowing for a more accurate dose delivery to the planning target volume.1,2 Tracking organs at risk (OARs) and the targeted lesion’s motion reduces margins of treatment volume and toxicity to normal tissues, leading to escalated treatment dose delivered to tumors and hence better treatment outcomes.In this initial study, we demonstrated the effectiveness of a motion correction technique and a deep-learning reconstruction developed previously at 3T on the 0.35T ViewRay System despite the low SNR.
Methods
A respiratory phantom and three healthy subjects, upon the approval of our Institutional Review Board, were imaged on the ViewRay system.3 The acquisition parameters for the T1-weighted self-navigated free breathing MRI motion correction method (CAPTURE: Consistently acquired projections for turned and robust estimation)4 were as follows: TE =2.31-2.33 ms, TR=4.55-4.99ms, FOV=350 x 350 - 360 x 360 mm2, voxel size=1.1x 1.1 x 3mm3, matrix size = 320 x 320 x 96, temporal resolution=176.3ms. 2000 radial spokes were acquired for 5 minutes 52 sec.The k-space data was binned into 5 respiratory phases with respect to the respiration curve derived by CAPTURE. Three image reconstruction methods were used to obtain 4D respiratory motion-resolved images including: (1) multi-coil non-uniform inverse fast Fourier Transform (MCNUFFT)5; (2) Compressed Sensing (CS)6; and (3) Phase2Phase (P2P),7 a deep learning reconstruction. Inspired by the Noise2Noise network8, a P2P network was trained without a high-quality ground truth as the target, i.e., using noisy 3T CAPTURE MCNUFFT images. Moreover, uncorrected NUFFT9 (non-MoCo) was performed to provide a reference.
For each of the 4D reconstruction methods, non-linear registration was performed to register all respiratory phases to the end-of-expiration phase to obtain 3D deformable motion vector fields (MVFs).
Results
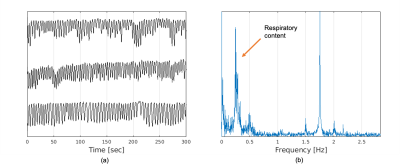
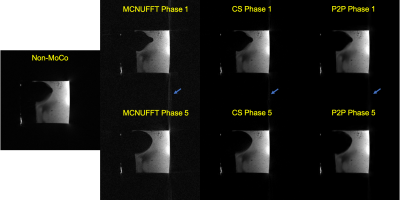
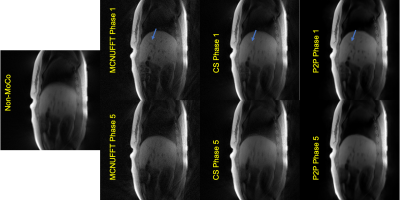
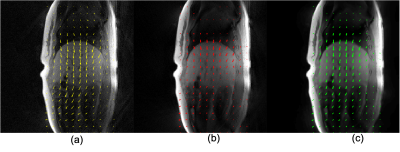
Figure 1 shows the detected respiratory curves from three healthy subjects and the frequency spectrum for one of the healthy subjects. Figure 2 demonstrates different reconstruction schemes (MCNUFFT, CS, P2P) for a respiratory motion phantom. As shown by the blue arrows, reduced artifacts can be observed on P2P images. Figure 3 exhibits different reconstruction results (MCNUFFT, CS, P2P) for a healthy subject. As shown by the blue arrows, CS images have fewer streaking artifacts than the MCNUFFT images but are blurrier. P2P images, on the other hand, are sharper and have fewer artifacts than the CS images.Deformable MVFs between the end-of-expiration and the end-of-inspiration were derived from 4D motion-resolved MR images reconstructed using MCNUFFT, CS and P2P. Figure 4 shows different MVFs (MCNUFFT, CS, P2P) overlaid onto the anatomy of a healthy subject. The average MVF motion vector length inside a ROI encompassing the entire liver is 9.49mm, 4.06mm and 6.37mm on MCNUFFT, CS and P2P images, respectively, which implies that CS reconstruction artificially reduces the motion range when compared to MCNUFFT. In contrast, P2P, despite being trained using noisy 3T MCNUFFT images, was able to better preserve the motion range when compared to CS images.
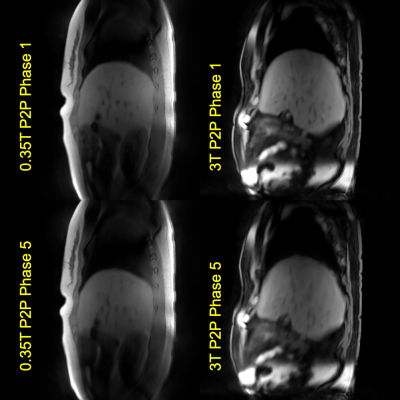
Figure 5 shows sample P2P images obtained at 0.35T MR vs at 3T. CAPTURE accompanied by P2P can still achieve similar image quality at 0.35T
Discussion and Conclusion
CAPTURE worked well at 0.35T and accurate respiratory motion information was obtained. Figures 2-3 demonstrate superior results with P2P when compared to MCNUFFT or CS: P2P outperforms CS in terms of sharpness and artifact. In addition, once trained, P2P reconstruction is fast. It only takes 17 seconds for a trained P2P network to reconstruct 4D MR data of a matrix size of 320x320x96x5 on a computer equipped with NVIDIA Tesla P100 GPU, whereas CS reconstruction takes 7 hours on the same dataset using an Intel Xeon E5-2690v4 Broadwell-EP 2.6-Ghz CPU.The proposed method can be adopted for low-field MRI in general, as there is renewed interest among the MRI community in using low-field MRI due to its reduced cost, smaller safety risks and easier maintenance.10,11 Currently, CAPTURE and 3T-trained P2P provide promising results on 3D deformation field derivation. Although these methods were initially developed at 3T, the results are promising for stereotactic cardiac MRI-guided radiosurgery. The T1W CAPTURE is much less sensitive than the current ViewRay TrueFISP sequences to banding artifacts from implanted cardiac devices typically found in the patient population.12 In the future, the P2P network will be re-trained using 0.35T images, potentially resulting in further improvements.
Acknowledgements
This study is partially supported by NIH 1R01HL148210 and Siemens Healthineers.References
1. Corradini, S. et al. MR-guidance in clinical reality: current treatment challenges and future perspectives. Radiation Oncology 14, 1–12 (2019).
2. Bortfeld, T., Jiang, S. B. & Rietzel, E. Effects of motion on the total dose distribution. in Seminars in radiation oncology vol. 14 41–51 (2004).
3. Mutic, S. & Dempsey, J. F. The ViewRay system: magnetic resonance--guided and controlled radiotherapy. in Seminars in radiation oncology vol. 24 196–199 (2014).
4. Eldeniz, C. et al. CAPTURE: Consistently Acquired Projections for Tuned and Robust Estimation: A Self-Navigated Respiratory Motion Correction Approach. Investigative radiology 53, 293–305 (2018).
5. Feng, L. et al. Golden-angle radial sparse parallel MRI: combination of compressed sensing, parallel imaging, and golden-angle radial sampling for fast and flexible dynamic volumetric MRI. Magnetic resonance in medicine 72, 707–717 (2014).
6. Lustig, M., Donoho, D. L., Santos, J. M. & Pauly, J. M. Compressed sensing MRI. IEEE signal processing magazine 25, 72–82 (2008).
7. Eldeniz, C. et al. Phase2Phase: Reconstruction of free-breathing MRI into multiple respiratory phases using deep learning without a ground truth. in Proc Intl Soc Magn Reson Med 28 0807 (Proc Intl Soc Magn Reson Med 28, 2020).
8. Lehtinen, J. et al. Noise2noise: Learning image restoration without clean data. arXiv preprint arXiv:1803.04189 (2018).
9. Fessler, J. A. & Sutton, B. P. Nonuniform fast Fourier transforms using min-max interpolation. IEEE transactions on signal processing 51, 560–574 (2003).
10. Campbell-Washburn, A. E. et al. Opportunities in interventional and diagnostic imaging by using high-performance low-field-strength MRI. Radiology 293, 384–393 (2019).
11. Marques, J. P., Simonis, F. F. J. & Webb, A. G. Low-field MRI: An MR physics perspective. Journal of magnetic resonance imaging 49, 1528–1542 (2019).
12. Gach, H. M. et al. Lessons learned from the first human low-field MRI guided radiation therapy of the heart in the presence of an implantable cardiac defibrillator. Practical radiation oncology 9, 274–279 (2019).
Figures