4240
Optimizing the use of diffusion tensor imaging for clinical tractography of the anterior cruciate ligament in the knee1School of Medicine, Queen's University, Kingston, ON, Canada, 2Center for Neuroscience Studies, Queen's University, Kingston, ON, Canada, 3Orthopedic Surgery, Queen's University, Kingston, ON, Canada, 4Diagnostic Radiology, Queen's University, Kingston, ON, Canada, 5Orthopedic Surgery, McGill University, Montreal, QC, Canada
Synopsis
Emergence of diffusion tensor imaging (DTI) has provided an exciting avenue to characterize the ligamentization process of the anterior cruciate ligament (ACL), following knee injury. However, a diverse, but limited, suite of published diffusion weighted sequences with varying parameters have posed a significant challenge to clinician-scientists wanting to integrate DTI as a tool to study the ACL. In this study, we provide a detailed comparison of DTI acquisitions toward the optimisation of ACL reconstruction using semi-automated fiber-based probabilistic tractography (FBPT), as a step-stone and reference for the clinical integration of DTI toward assessing microstructural integrity of the cruciate ligaments.
INTRODUCTION
Conventional MRI is used qualitatively to assess the integrity of the anterior cruciate ligament (ACL),1 although it has limited value with respect to clinical predictions post-ACL reconstruction. Recently, diffusion tensor imaging (DTI) has emerged as a novel method to expose changes in the maturation of the ACL graft postoperatively,3–6 or following an injury,7 providing an non-invasive biomarker for assessing directional anisotropy within the ligament, as a substrate for microstructural integrity.8,9Given its novelty as a biomarker for studying the ACL, only a limited sample of papers have been published using a spectrum of sequence parameters for diffusion weighted imaging (i.e. different number of slices, directions, b-values, spatial resolution, etc.),3,6 which poses a significant challenge for new clinician-scientists looking to incorporate the use of DTI as a tool to study the ACL. Here, we compare differences in DTI acquisitions for optimising segmentation of the ACL using semi-automated fiber-based probabilistic tractography (FBPT),10,11 as a step-stone and reference for the clinical integration of DTI to assess microstructural integrity of the cruciate ligaments.
METHODS
All images were acquired on a Magnetom Prisma Siemens scanner at 3.0T, using a secured flexible 18-channel body coil wrapped around the knee. A total of three diffusion sequences (DTI A, B, C) were acquired (Table 1; Figure 1). Sequence A was replicated based on existing literature,5,6 while sequences B and C were attempts to improve the original acquisition using simultaneous image refocusing (multiband), which is known to drastically reduce acquisition time, while allowing for a greater number of gradient directions and higher spatial resolution. Sequences B and C were compared directly to assess for the effect of acquiring multiple b-values, which is a major difference noted in the existing literature.6,7 This is likely due to the longer echo times required with adding additional b acquisitions, which is sub-optimal for ligamentous imaging.12,13A 3D Double Echo Steady State (DESS) sequence with isotropic resolution (8.35 min; in-plane res., 0.2 x 0.2 mm ; slice thickness, 0.4 mm; TR/TE, 14.16/5.00 ms; Partial Fourier, 7/8) was acquired (Figure 2) on which 15 mm spherical regions of interest (ROI) were placed consistent with the end points of the ACL (femur, posteromedial corner on medial aspect of lateral condyle; tibia, anteromedial to intercondylar eminence) (Figure 2). The ACL was also manually segmented to assess the performance of each FBPT reconstruction using Dice Coefficients (Figure 2).
All diffusion images were processed using the FMRIB FSL toolbox14 which included: affine linear registration to the initial b0 image, averaging, estimation and correction of susceptibility and eddy-current induced distortions, and voxelwise tensor calculation. Transformation matrices coregistering b0 images to the high-resolution anatomical image (6 DOF) were inverted to align the ROIs for FBPT into native diffusion space.
Following Bayesian estimation of diffusion parameters and modelling of crossing fibers,15 the ACL for each DTI sequence was reconstructed using FBPT.10,11 This was repeated twice using each spherical ROI as the seed, or a target.5,6 The final tractography-based ACL mask was constructed in two ways, once using voxels only traversed by both tractograms,5,6 and second, using voxels traveled by either tractograms.
Acquisition methods were assessed for signal-to-noise ratio (SNR = meansignal(acl)/stdsignal(background)), which was extracted from the manually segmented ACL (Figure 2) for consistency. Mean fractional anisotropy (FA) was extracted from all tractography-derived ACLs for direct comparison of each methods. Lastly, a Dice Coefficient for each FBPT-ACL was computed against the reference ACL, in order to assess for each method’s ability to reconstruct the ligament.
RESULTS/DISCUSSION
SNR analysis revealed that both A and C acquisitions averaged higher SNR than B (Figure 3). This is expected given the higher spatial resolution and shorter echo time employed in B (Table 1), which is essential to optimize ligamentous imaging.12,13 No tractograms could be reconstructed from the original sequence (A, Figure 4). This may due to the challenges associated with reconstructing the ACL in healthy controls using this sequence, as recognized by the original authors.5,6 This suggests that both protocols B and C may provide superior acquisitions for semi-automated ACL segmentation using tractography.Dice Coefficient analysis highlighted protocol B is the preferred acquisition for reconstruction of the ACL, given that it provides a balanced compromise between higher spatial resolution, and lower echo time (Table 1; Figure 4). Moreover, segmentation of the ACL using voxels traveled by either tractograms also improved the final mask (Figure 4, right), irrespective of the sequence used, which is another important methodological improvement from the original methods.5–7 Lastly, direct comparison of the FA measurements for each sequence suggests comparable estimates of anisotropy within the ligament, in line with reported values (Table 2).5–7
CONCLUSION
The current analysis suggests that DTI protocol B is optimal for the clinical use of diffusion imaging as a tool to assess the microstructural integrity of the ACL. This is because it provides a balanced compromise between higher spatial resolution, lower echo time, shorter scan time and improved resultant FBPT-ACL segmentation. This pilot offers an important milestone for the integration of DTI as a tool to study the ACL, putting forward a complete protocol with high-resolution 3D structural imaging that can be completed within ~12 minutes.Acknowledgements
No acknowledgement found.References
1. Van Dyck P, Zazulia K, Smekens C, Heusdens CHW, Janssens T, Sijbers J. Assessment of Anterior Cruciate Ligament Graft Maturity With Conventional Magnetic Resonance Imaging: A Systematic Literature Review. Orthop J Sport Med. Published online 2019. doi:10.1177/2325967119849012
2. Saupe N, White LM, Chiavaras MM, et al. Anterior cruciate ligament reconstruction grafts: MR imaging features at long-term follow-up-correlation with functional and clinical evaluation. Radiology. Published online 2008. doi:10.1148/radiol.2492071651
3. Yang X, Li M, Chen D, et al. Diffusion tensor imaging for anatomical and quantitative evaluation of the anterior cruciate ligament and ACL grafts: A preliminary study. J Comput Assist Tomogr. 2014;38(4):489-49
4. doi:10.1097/RCT.00000000000000784. Yang X, Chen D, Li M, Shi D, Zhu B, Jiang Q. Diffusion tensor imaging of the anterior cruciate ligament graft after reconstruction: Repeatability and diffusion tensor imaging metrics. J Comput Assist Tomogr. Published online 201
5. doi:10.1097/RCT.00000000000001985. Van Dyck P, Froeling M, De Smet E, et al. Diffusion tensor imaging of the anterior cruciate ligament graft. J Magn Reson Imaging. 2017;46(5):1423-1432. doi:10.1002/jmri.25666
6. Van Dyck P, Froeling M, Heusdens CHW, Sijbers J, Ribbens A, Billiet T. Diffusion tensor imaging of the anterior cruciate ligament following primary repair with internal bracing: A longitudinal study. J Orthop Res. 2020;(March):1-13. doi:10.1002/jor.24684
7. Liu S, Liu J, Chen W, et al. Diffusion Tensor Imaging for Quantitative Assessment of Anterior Cruciate Ligament Injury Grades and Graft. J Magn Reson Imaging. Published online 2020:1-10. doi:10.1002/jmri.27322
8. Johansen-Berg H, Behrens TEJ. Diffusion MRI: From Quantitative Measurement to in-Vivo Neuroanatomy.; 200
9. doi:10.1016/B978-0-12-374709-9.00002-X9. Dong S, Xie G, Zhang Y, Shen P, Huangfu X, Zhao J. Ligamentization of Autogenous Hamstring Grafts after Anterior Cruciate Ligament Reconstruction: Midterm Versus Long-term Results. Am J Sports Med. 2015;43(8). doi:10.1177/0363546515584039
10. Behrens TEJ, Berg HJ, Jbabdi S, Rushworth MFS, Woolrich MW. Probabilistic diffusion tractography with multiple fibre orientations: What can we gain? Neuroimage. 2007;23. doi:10.1016/j.neuroimage.2006.09.018
11. Behrens TEJ, Woolrich MW, Jenkinson M, et al. Characterization and Propagation of Uncertainty in Diffusion-Weighted MR Imaging. 2003;1088:1077-1088. doi:10.1002/mrm.10609
12. Wengler K, Tank D, Fukuda T, et al. Diffusion tensor imaging of human Achilles tendon by stimulated echo readout-segmented EPI (ste-RS-EPI). Magn Reson Med. 2018;80(6):2464-2474. doi:10.1002/mrm.27220
13. Wang N, Mirando AJ, Cofer G, Qi Y, Hilton MJ, Johnson GA. Diffusion tractography of the rat knee at microscopic resolution. Magn Reson Med. 2019;81(6):3775-3786. doi:10.1002/mrm.27652
14. Jenkinson M, Beckmann CF, Behrens TEJ, Woolrich MW, Smith SM. Fsl. Neuroimage. 2012;62(2):782-790. doi:10.1016/j.neuroimage.2011.09.015
15. Jbabdi S, Sotiropoulos SN, Savio AM, Graña M, Behrens TEJ. Model-based analysis of multishell diffusion MR data for tractography: How to get over fitting problems. Magn Reson Med. Published online 2012. doi:10.1002/mrm.24204
16. Griswold MA, Jakob PM, Heidemann RM, et al. Generalized autocalibrating partially parallel acquisitions (GRAPPA). Magn Reson Med. Published online 2002. doi:10.1002/mrm.10171
Figures
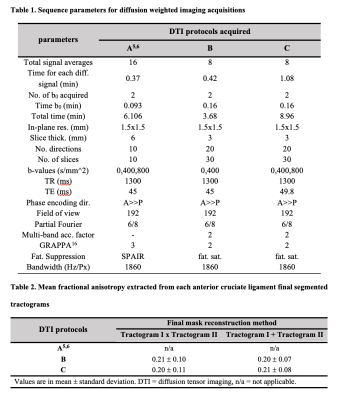
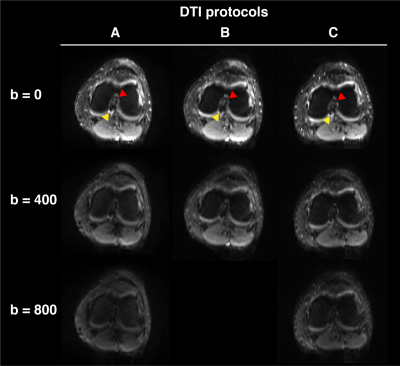
Figure 1. Diffusion-weighted scans of the left knee
Single slices for each diffusion weighted scans acquired (protocols A, B and C; see Table 1) highlight the anterior (red arrow) and posterior (yellow arrow) cruciate ligaments with minimal distortions due to susceptibility artifacts within the intercondylar notch and adequate signal. The different diffusion weightings (b = 0, 400, 800 s/mm2) are shown for each acquisition, when available.
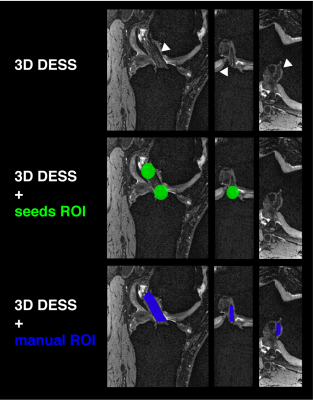
Figure 3. Three-dimensional high resolution structural imaging of the left knee
Three views (sagittal, coronal and axial) of the Double Echo Steady State (DESS) scan are shown highlighting the healthy anterior cruciate ligament (ACL) for this patient (top). The middle and bottom views show the placement of the region of interest seeds within the tibia and femur insertions of the ACL (green), as well as the manual segmentation of the ligament (blue) used for assessment of the tractography outputs.
Figure 4. Signal to noise analysis
The signal to noise ratio (SNR) is plotted for each diffusion gradient directions, for each diffusion-weighted imaging protocol acquired (A, B and C; see Table 1). The volume 0 represents the b0 volume, for each acquisition, hence the higher SNR across each plot. The red dotted line represents the mean SNR for that protocol. (a) DTI A, (b) DTI B and (c) DTI C.
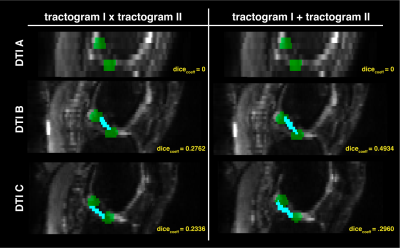
Figure 5. Results from the fiber-based probabilistic tractography and Dice Coefficient analysis
Sagittal slices of the diffusion weighted images (grayscale) are displayed and overlaid with the resampled regions of interest (green, see Figure 2), as well as the reconstructed anterior cruciate ligament (cyan blue), estimated based on fiber-based probabilistic tractography. Both segmentation reconstructions are shown including voxels traversed by the two (left), or either (right), tractograms.