4216
Quantitative Magnetization Transfer MRI of the Knee Meniscus: Correlations with Biochemistry and Histology1Biomedical Engineering, University of Saskatchewan, Saskatoon, SK, Canada, 2Mechanical Engineering, University of Saskatchewan, Saskatoon, SK, Canada, 3Medical Physics, McGill University, Montreal, QC, Canada
Synopsis
The objective of this research was to determine if quantitative magnetization transfer (qMT) MRI can assess knee meniscus tissue health. Cadaver knee menisci underwent qMT assessment, biochemical content assessment, and histological scoring. Weak correlations were found between T1f and collagen content, T1f and histology score, and the histology scores and T2f and T2b. A moderate correlation was found between T2f and collagen content. qMT shows promise as a measure of meniscal tissue health but future studies with more severely damaged tissue are required to elucidate relationships between qMT and measures of meniscal content and health.
Introduction
The meniscus plays an essential role in knee joint health and function. With joint diseases such as osteoarthritis, macromolecular structure degrades, and meniscus function is compromised. Quantitative magnetization transfer (qMT) MRI is particularly well suited to study meniscus in healthy and diseased states because it probes protons bound to macromolecules (which in meniscus is predominantly proteoglycans and collagen) and free protons within the tissue. We expect to see changes in qMT parameters with degradation in the meniscus. Previous studies in cartilage showed moderate correlations between qMT parameters and biochemical properties1 and differences between osteoarthritis patients and healthy individuals2; but only proof of concept has been shown in the meniscus3. The use of qMT in the meniscus requires further validation. The objective of this work is to identify correlations between qMT parameters f (bound water fraction), k (magnetization exchange rate), T2b (T2 relaxation time of the bound pool), T2f (T2 relaxation time of the free pool), and T1f (T1 relaxation time of the free pool) and proteoglycan content, collagen content, and histological score of human cadaver menisci.Methods
Six cadaver knees with no history of injury or surgery (3 males, 3 females, mean age 70.3 ± 9.3) were scanned in situ at 3T (MAGNETOM Skyra, Siemens, Erlangen, Germany) using an in-house spoiled gradient-recalled echo (SPGR) sequence with pulsed off-resonance saturation for MT contrast and an 18-channel flexible receive coil. The protocol included 10 MT-SPGR scans (five offset frequencies: 433, 1087, 2732, 6862, 17235 Hz, at two flip angles: 142˚ and 426˚) and one (MT-disabled) SPGR scan, all with field of view=160×160mm2, TR/TE=48/3ms, matrix size=256×256, and slice thickness=3mm. B1 (assessed using a standard double angle technique4) and T1 (obtained with driven equilibrium single pulse observation of T1 (DESPOT1)5) maps were acquired for correction and qMT-modeling, respectively. B0 correction was not performed because of the small variation in B0 across the meniscus. By assuming T1 of the bound pool (T1b) to be 1 s and using the T1 maps, a two-pool model of MT with Gaussian lineshape was fit and used to estimate qMT parameters6-8. After scanning, menisci were dissected, and a 4 mm biopsy punch was used to procure cylindrical samples for biochemical analysis. Menisci were cut radially into seven roughly equal pieces and every second used in histological analysis representing anterior, central, and posterior regions. Cylindrical samples were weighed, vacuum dried, weighed to determine liquid content, and digested using proteinase K. Proteoglycan and collagen content were assessed using a dimethylmethylene blue (DMMB) assay (sulfated GAG as surrogate) and hydroxyproline assay kit (Sigma Aldrich, MAK008), respectively. Radial pieces were fixed, paraffin embedded, sectioned, stained using an established safranin O/fast green protocol9, then scored with a modified meniscus specific histology scoring system10. qMT, biochemistry, and histology data were registered using a custom image processing pipeline and relationships identified using a Pearson product moment correlation for biochemical properties and Spearman’s correlation test for histological scores (SPSS, Chicago, IL, USA).Results
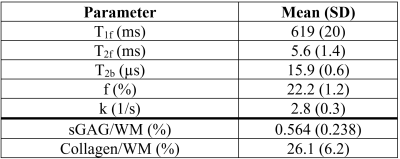
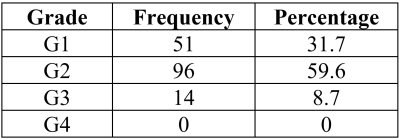
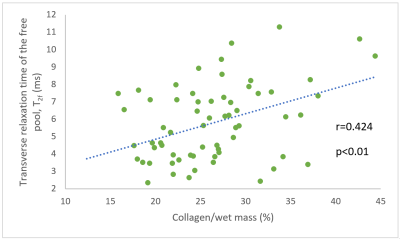
Mean qMT and biochemical results were obtained for the menisci of the six specimens and 70 cylindrical samples, respectively (Table 1). Histological scoring was obtained for 161 sections (Table 2). Significant correlations of r=0.318 (p < 0.01) and r=0.424 (p < 0.01) were found between collagen per wet mass (WM) and T1f and T2f relaxation times, respectively (Figure 1). Significant correlations of ρ=-0.232 (p<0.05), ρ=-0.277 (p<0.01), and ρ=-0.207 (p<0.05) were found between total histological score and T1f, T2f, and T2b relaxation times, respectively. No significant correlations were observed between any tissue properties measured and f or k.Discussion
Average values for qMT parameters align with previous results in the meniscus3, except T2b relaxation time in which our values were higher. This is very likely due to differences in the fitting algorithm; we used a Gaussian lineshape as it consistently resulted in slightly better fits than the super-Lorentzian lineshape (r2=0.91±0.03 and r2=0.89±0.03, respectively). Of note, there was a difference in k due to this choice; however, use of a Gaussian lineshape for qMT modeling has been reported for soft tissues7. Further studies should be done to determine which lineshape is most appropriate for the meniscus. In general, the meniscus had higher f, lower k, and higher T2b as compared to articular cartilage values in literature1,2. The correlation strengths observed may be limited by the small range of tissue health in our samples. From the histological scoring, it can be noted that health of the cadaver specimens was relatively homogeneous (over 90% of samples had a histological grade of 1 or 2), and this may be why stronger correlations were not observed. The correlations observed indicate that the qMT parameters appear to be more related to the state (histology) and collagen content rather than amount of proteoglycan (sGAG). Future studies including more diseased tissue will elucidate relationships between qMT parameters and biochemical content and histological scores in the meniscus.Conclusion
Preliminary findings suggest that qMT may be useful for assessing meniscus health, however, further work using tissues with greater variation in disease state is required to better characterize the qMT – tissue health relationship.Acknowledgements
The authors would like to acknowledge The Arthritis Society Young Investigator Operation Grant, Natural Sciences and Engineering Research Council Discovery Grant, MITACS Accelerate, Siemens Healthineers, and University of Saskatchewan Devolved Scholarship Program.References
1. Stikov N, Keenan KE, Pauly JM, Smith RL, Dougherty RF, Gold GE. Cross-relaxation imaging of human articular cartilage. Magn Reson Med. 2011;66(3):725-734. doi:10.1002/mrm.22865
2. Sritanyaratana N, Samsonov A, Mossahebi P, Wilson JJ, Block WF, Kijowski R. Cross-relaxation imaging of human patellar cartilage in vivo at 3.0T. Osteoarthr Cartil. 2014;22(10):1568-1576. doi:10.1016/j.joca.2014.06.00
3. Simard M, McWalter E, Gold G, Levesque I. Quantitative magnetization transfer MRI of in-situ and ex-situ meniscus, Proceedings of the ISMRM, 2016, p.2266
4. Insko EK, Bolinger L. Mapping of the radiofrequency field. J Magn Reson - Ser A. 1993;103(1):82-85. doi:10.1006/jmra.1993.1133
5. Deoni S, Peters T, Rutt, B. High-resolution T1 and T2 mapping of the brain in a clinically acceptable time with DESPOT1 and DESPOT2. Magn Reson Med, vol. 5, pp.237-41, Jan 2005.
6. Simard M. Quantitative magnetization transfer evaluation of the knee joint in magnetic resonance imaging, Master of Science, McGill University, 20165.
7. Ramani, C. Dalton, D. H. Miller, P. S. Tofts, and G. J. Barker, "Precise estimate of fundamental in-vivo MT parameters in human brain in clinically feasible times," Magn Reson Imaging, vol. 20, pp. 721-31, Dec 2002.
8. Sinclair, C.D., Samson, R.S., Thomas, D.L., Weiskopf, N., Lutti, A., Thornton, J.S., et al., "Quantitative magnetization transfer in in vivo healthy human skeletal muscle at 3 T," Magn Reson Med, vol. 64, pp. 1739-48, Dec 2010.
9. Schmitz N, Laverty S, Kraus VB, Aigner T. Basic methods in histopathology of joint tissues. Osteoarthr Cartil. 2010;18:S113-S116. doi:10.1016/j.joca.2010.05.026
10.
Pauli C, Grogan SP, Patil S, et al. Macroscopic and histopathologic analysis of
human knee menisci in aging and osteoarthritis. Osteoarthr Cartil. 2011;19(9):1132-1141.
doi:10.1016/j.joca.2011.05.008
Figures