4201
Optimized DANTE preparation for intracranial DANTE-SPACE vessel wall imaging at 7T1Wellcome Centre for Integrative Neuroimaging, FMRIB Division, Nuffield Department of Clinical Neurosciences, University of Oxford, Oxford, United Kingdom, 2Oxford Centre for Clinical Magnetic Resonance Research, Department of Cardiovascular Medicine, University of Oxford, Oxford, United Kingdom
Synopsis
DANTE-SPACE can be used for visualizing intracranial vessel walls at 7T by simultaneously suppressing signal from the luminal blood and external CSF. However, vessel wall delineation is limited by the achieved contrast and resolution, and further improvement of the sequence is constrained by SAR. Here, an optimized protocol for the number of DANTE-pulses and the flip angle of each DANTE-pulse is proposed based on an EPG-based simulation framework. In-vivo data acquired using this DANTE preparation show an improved CNR and signal ratio between the vessel wall and both blood and CSF, while reducing the SAR compared to previous DANTE preparations.
Introduction
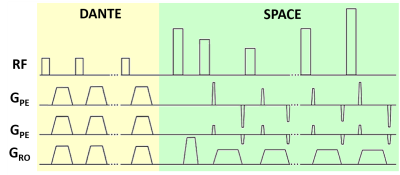
Vessel wall imaging (VWI) can be used to assess the pathology of intracranial vessel walls. For visualization of the middle cerebral arteries (MCAs), the signal from both the internal lumen and the external CSF needs to be suppressed. For this, a previous study presented a DANTE-SPACE sequence at 7T1. DANTE-SPACE (Figure 1) achieves delineation of the MCA walls by employing an optimized variable-flip-angle turbo spin echo sequence (SPACE2) with additional DANTE-preparation3 for flow-sensitive signal suppression of both the CSF and the lumen. This results in a sharper vessel wall point-spread-function (PSF) and increased contrast between the vessel wall and CSF. However, vessel wall delineation is limited by the achieved contrast and resolution, and sequence development is constrained by high SAR values1.In this work, we developed a framework to simulate the achieved lumen-, CSF-, and vessel wall-signal for a DANTE-SPACE sequence at 7T. This framework is used to optimise the achieved CNR by adapting the sequence parameters and the identified parameters were then evaluated in vivo.
Methods
DANTE-SPACE was simulated using the extended phase graph (EPG) framework4,5. For validation, simulations for the DANTE-preparation were compared against literature single isochromat results3, and the SPACE-readout simulation for the case of stationary spins was cross-checked against the target SPACE magnetization function1,2.In addition to the tissue-dependent relaxation properties, the simulations included flow velocity distributions, pulsatile velocity variations6, and typical flow trajectories. B1+-variations in different parts of the vasculature based on measured in vivo B1+ maps were included to facilitate a comprehensive assessment of the efficacy of the DANTE-SPACE sequence.
The contrast between tissue types was calculated from the PSF amplitude of the transverse magnetization during the SPACE-readout for the various tissue types after correcting for differences in proton density. Each tissue type was simulated using different relaxation and flow properties1,6,7. For the vessel wall and CSF, constant B1+-signal was assumed because of their limited spatial displacement. For non-static tissues, signals were averaged over 100 simulations with velocities ranging between the mean value ± 10%. Results from this simulation framework were used to propose the optimal sequence parameters that lead to the highest CNR and ratio between the signal intensities (signal ratio; SR) between the vessel wall and both the blood and CSF.
Data were acquired on a Siemens Magnetom 7T scanner (Erlangen, Germany) using a 1Tx, 32-channel Rx head-coil from 5 healthy volunteers (ages 22-55, 1 female). Two VWI datasets were acquired, one with the “optimised” DANTE train as determined by the EPG simulations and one based on the “standard” acquisition parameters of Viessmann et al.1. The latter parameters included 300 DANTE-pulses of 10o, and a SPACE readout consisting of 75 pulses with variable FAs and an echo spacing of 4.6ms. The overall TR for each readout was 2.64s. The acquisition time was approximately 11 minutes using a GRAPPA-factor of 4. All images were acquired with a resolution of 0.5x0.5x1.0 mm3 and reconstructed to 0.25x0.25x1.0 mm3. In three volunteers a dataset without any DANTE preparation was also acquired.
Results
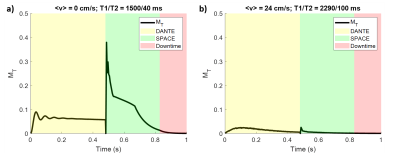
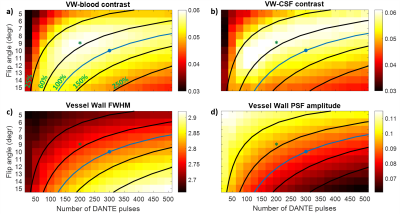
Figure 2 shows the simulated transverse magnetization of spins in the vessel wall and the blood in a DANTE-SPACE acquisition.Figure 3 shows the simulation results using different numbers of DANTE pulses and different DANTE flip angles. Figures 3a-b show the resulting contrast of the vessel wall with the blood and CSF. Figures 3c-d show the resulting FWHM and amplitude of the vessel wall PSF.
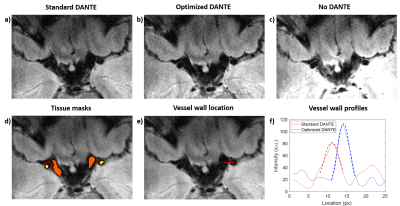
An increase in vessel wall contrast is found when using 200 DANTE preparation pulses of 9o (“optimized”) which also reduced the SAR compared with previous work1. Figures 4a-c show a slice from a DANTE-SPACE acquisition in a healthy volunteer using the “standard” DANTE and the “optimized” DANTE, as well as when using SPACE without any DANTE preparation. The vessel wall sharpness is compared using the vessel wall profiles, in Figures 4e-f.
Figure 5 shows that the CNR and the signal ratio between the vessel wall and both the CSF (CNR +23.8%, SR +10.5%) and the lumen (CNR + 22.6%, SR +9.1%), as calculated using the masks in Fig. 4d, increase when using “optimized” DANTE-preparation, while the vessel wall sharpness remains constant. Note that this is achieved with a reduced SAR compared to “standard” DANTE1.
Discussion
A simulation-based optimization of DANTE-SPACE for VWI at 7T indicates that reducing the flip angle and number of pulses in the DANTE-preparation improves the vessel wall contrast, thus also reducing the SAR and maintaining good vessel wall sharpness. This result was confirmed when comparing the different preparations in vivo. Although the SNR of all tissue types increases when using the “optimized” DANTE preparation, this increase is greater for the vessel wall signal, resulting in an increased signal ratio between vessel wall and the CSF/blood.A reduction in SAR can facilitate further improvements to the DANTE-SPACE sequence at 7T, such as a reduction in acquisition time or increase in resolution by sampling a reduced field-of-view while employing additional spatial saturation pulses.
Conclusion
Reducing the number of pulses and the flip angle of the DANTE-preparation of DANTE-SPACE at 7T increases the vessel wall contrast in the MCAs while reducing the SAR of the sequence.Acknowledgements
MdB receives financial support from Siemens Healthineers and the Dunhill Medical Trust.References
[1] O. Viessmann, L. Li, P. Benjamin, and P. Jezzard, “T2-Weighted intracranial vessel wall imaging at 7 Tesla using a DANTE-prepared variable flip angle turbo spin echo readout (DANTE-SPACE),” Magn. Reson. Med., vol. 77, no. 2, pp. 655–663, 2017.
[2] J. P. Mugler, “Optimized three-dimensional fast-spin-echo MRI,” J. Magn. Reson. Imaging, vol. 39, no. 4, pp. 745–767, 2014.
[3] L. Li, K. L. Miller, and P. Jezzard, “DANTE-prepared pulse trains: A novel approach to motion-sensitized and motion-suppressed quantitative magnetic resonance imaging,” Magn. Reson. Med., vol. 68, no. 5, pp. 1423–1438, 2012.
[4] J. Hennig, “Multiecho imaging sequences with low refocusing flip angles,” J. Magn. Reson., vol. 78, no. 3, pp. 397–407, 1988.
[5] M. Weigel, “Extended phase graphs: Dephasing, RF pulses, and echoes - Pure and simple,” J. Magn. Reson. Imaging, vol. 41, no. 2, pp. 266–295, 2015.
[6] A. D. Augst, D. C. Barratt, A. D. Hughes, S. A. McG Thom, and X. Y. Xu, “Various issues relating to computational fluid dynamics simulations of carotid bifurcation flow based on models reconstructed from three-dimensional ultrasound images,” Proc. Inst. Mech. Eng. Part H J. Eng. Med., vol. 217, no. 5, pp. 393–403, 2003.
[7] T. Wada et al., “Visualization of cerebrospinal fluid dynamics using multi-spin echo acquisition cine imaging (MUSACI),” Magn. Reson. Med., vol. 81, no. 1, pp. 331–341, 2019.
Figures