4195
Pure balanced steady-state free precession (bSSFP) imaging1Department of Biomedical Engineering, University of Basel, Basel, Switzerland, 2Department of Radiology, Division of Radiological Physics, University Hospital Basel, Basel, Switzerland, 3Department of Diagnostic and Interventional Radiology, Klinikum rechts der Isar, Technical University of Munich, Munich, Germany
Synopsis
For some tissues bSSFP shows a distinct asymmetry in its frequency response function. Theoretical considerations indicate that this asymmetry disappears in the limit of $$$\mathit{TR} \rightarrow 0$$$. This was studied experimentally at $$$3\,\mathrm{T}$$$ in the present work. The frequency response of bSSFP was investigated for in vivo brain for varying repetition time $$$\mathit{TR} = \{1.5,3,5\}\,\mathrm{ms}$$$. Our results give strong evidence that the asymmetry vanishes in the limit of $$$\mathit{TR} \sim 1\,\mathrm{ms}$$$ and therefore indicate that bSSFP forgets about the spectral composition and thus becomes ''pure''.
Introduction
The concept of balanced steady-state free precession (bSSFP) has been first introduced by Carr1 in the late 1950s in the context of spectroscopy. Since then it has become increasingly popular for imaging due to its high signal efficiency and its $$$T_2/T_1$$$-weighted contrast.Although its signal properties have been extensively investigated, it was only noted about a decade ago by Miller2 that for bSSFP various tissues exhibit a strong and rather unexpected asymmetry in the frequency profile. Generally, the observed asymmetry in tissues arises from local intra-voxel frequency distributions driven by its rich and complex underlying microstructure. This effect is especially strong in brain tissue where myelination leads to a frequency dispersion. In this work, we use configuration theory to show that, generally, tissues forget about their spectral composition when using bSSFP imaging in the limit of $$$\mathit{TR}\rightarrow 0$$$. It is shown that this holds true for $$$\mathit{TR}\sim1\,\mathrm{ms}$$$ at $$$3\,\mathrm{T}$$$.
Theory
We consider the spin density $$$M$$$ being composed of a normalized spectrum of magnetizations $$$m_k$$$ with associated chemical shifts $$$\delta_k$$$. We now look at a train of RF pulses with equidistant spacing given by $$$\mathit{TR}$$$ and linear RF phase increase $$$\varphi_j=-j\phi$$$ for the $$$j$$$th $$$\mathit{TR}$$$ interval, where $$$\phi$$$ is the phase increase per $$$\mathit{TR}$$$. The magnetization immediately after the RF pulse can be expressed as a series over all configuration orders and spectral components:$$M(\phi,k)=\sum_{n,k} e^{in(\theta_k + \phi)}m_k^{(n)}\tag{1}$$
where $$$\theta_k=2\pi\gamma B_0\delta_k\mathit{TR}$$$ denotes the phase accumulation of $$$m_k$$$ within any $$$\mathit{TR}$$$ at some given main magnetic field strength $$$B_0$$$.
We immediately see that $$$\lim\limits_{\mathit{TR}\rightarrow 0}\theta_k=0$$$ and therefore
$$\lim\limits_{\mathit{TR}\rightarrow 0}M(\phi,k)=\sum_{n,k}e^{in\phi}m_k^{(n)}=\sum_{n=-\infty}^{\infty}e^{in\phi}\left(\sum_{k=0}^{K}m_k^{(n)}\right)=\sum_{n=-\infty}^{\infty}e^{in\phi}M^{(n)}=M(\phi).\tag{2}$$
This means the voxel eventually ''forgets'' about its spectral composition, having the apparent properties of a ''pure'' voxel. For the $$$2\pi$$$ periodicity of the bSSFP frequency response function the ''purity'' condition
$$2\pi\gamma B_0\delta_k\mathit{TR}\approx0\tag{3}$$
implies
$$\delta_k\ll\frac{1}{\gamma B_0\mathit{TR}}\hspace{.5cm}\mbox{or}\hspace{.5cm}\mathit{TR}\ll\frac{1}{\gamma B_0\delta_k}\label{limits}\tag{4}$$
Notably this becomes less stringent with decreasing $$$B_0$$$ for fixed $$$\delta_k$$$.
For brain tissue a frequency dispersion of about $$$20\,\mathrm{Hz}$$$ was observed at $$$3\,\mathrm{T}$$$ 2, leading to a limit of $$$\mathit{TR}\ll50\,\mathrm{ms}$$$. On modern scanners a $$$\mathit{TR}$$$ of $$$1-2\,\mathrm{ms}$$$, which is expected to fulfill the limit, can generally be reached for reasonable resolutions, therefore opening the possibility to validate the theory.
Methods
Imaging was performed at $$$3\,\mathrm{T}$$$ using a slightly modified standard cartesian bSSFP sequence with automated phase-cycling and optional reversed sweep direction. Scans were performed for pure spin densities using a manganese-doped, aqueous phantom $$$(T_1=860\,\mathrm{ms},T_2=70\,\mathrm{ms})$$$ and for in vivo brain where a strong profile asymmetry is expected3.Non-selective bSSFP imaging was performed with a nominal flip angle of $$$\alpha=10^{\circ}$$$ for three different values of $$$\mathit{TR}\,(1.5\,\mathrm{ms},3.0\,\mathrm{ms},5.0\,\mathrm{ms})$$$ with corresponding bandwidths of $$$\{1775,610,270\}\,\mathrm{Hz/Px}$$$. A field-of-view of $$$256$$$ with $$$75\%$$$ resolution in phase direction, a base resolution of $$$128$$$ and a slice thickness of $$$2.0\,\mathrm{mm}$$$ were chosen, resulting in an imaging matrix of $$$128\times96\times80$$$ and an isotropic resolution of $$$2.0\,\mathrm{mm}$$$. For each $$$\mathit{TR}$$$ setting, $$$N$$$ scans with equally distributed linear RF phase increments in the interval $$$[0,2\pi)$$$ were recorded. A dummy preparation period of $$$3-5\,\mathrm{s}$$$ was used to ensure steady-state conditions. The phantom scans were performed with $$$N = 72$$$, the brain scans with $$$N = 36$$$.
Scanning took $$$20\,\mathrm{min}$$$ $$$(\mathit{TR}=1.5\,\mathrm{ms}$$$ and $$$\mathit{TR}=3\,\mathrm{ms})$$$ and $$$24\,\mathrm{min}$$$ $$$(\mathit{TR}=5\,\mathrm{ms})$$$, respectively, for the phantom and $$$10\,\mathrm{min}$$$ $$$(\mathit{TR}=1.5\,\mathrm{ms}$$$ and $$$\mathit{TR}=3\,\mathrm{ms})$$$ and $$$12\,\mathrm{min}$$$ $$$(\mathit{TR}=5\,\mathrm{ms})$$$, respectively, for the brain scan to complete. For the brain scans the asymmetry index $$$(\mathit{AI})$$$ was calculated as stated in Miller et. al.3 via
$$\mathit{AI} = \frac{h_p-h_n}{h_p + h_n}\tag{5}$$
where $$$h_{p,n}$$$ is the peak signal at the positive ($$$p$$$) or negative ($$$n$$$) frequency offset from the center.
Results & Discussion
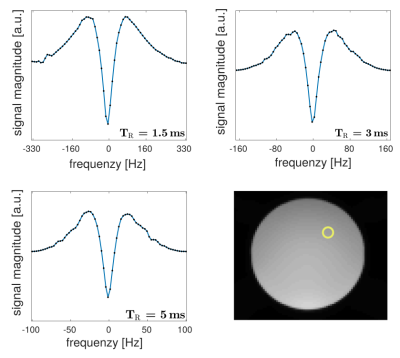
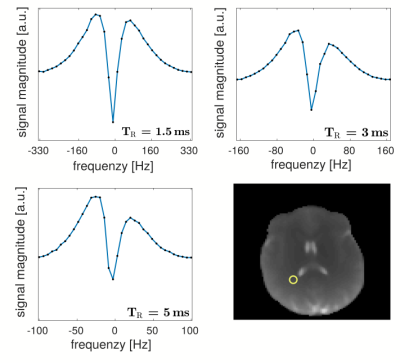
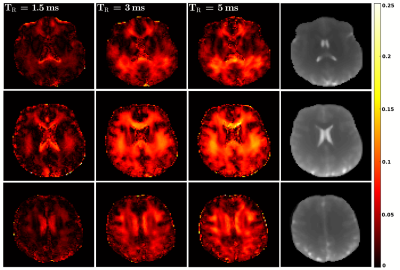
For the phantom scans (Fig. 1), the observed frequency response of bSSFP is symmetric for all $$$\mathit{TR}$$$, as can be expected for pure voxels with symmetric frequency dispersions. This is in contrast to the findings for brain white matter (Fig. 2). Here, a pronounced asymmetry is observed for a $$$\mathit{TR}$$$ of $$$5\,\mathrm{ms}$$$, in agreement with previous findings2. The asymmetry index, however, becomes markedly attenuated with a decrease in $$$\mathit{TR}$$$, as is evident from the figure. $$$\mathit{AI}$$$ maps are shown in Fig. 3 for three axial slices. A morphological image is shown as reference. As reported by Miller et. al.3 a rich variation of the $$$\mathit{AI}$$$ is observed for brain tissue, with highest $$$\mathit{AI}$$$ values in the corpus callosum, while the region around the corticospinal tract exhibits generally the lowest asymmetry. Overall, the $$$\mathit{AI}$$$ maps show a pronounced sensitivity on the $$$\mathit{TR}$$$ and decrease with decreasing $$$\mathit{TR}$$$, as expected from theory (please note that the cerebrospinal fluid would require at least three times $$$T_1$$$ to yield a symmetric response and thus the observed residual asymmetry reflects transient effects). Consequently, for a $$$\mathit{TR}$$$ of about $$$1\,\mathrm{ms}$$$, bSSFP imaging of brain tissue becomes apparently pure at $$$3\,\mathrm{T}$$$. This limit will become less stringent at lower fields (cf. Eq.4) and pure bSSFP imaging of tissues will become feasible with clinically relevant resolutions, e.g. at $$$1.5\,\mathrm{T}$$$ using a $$$\mathit{TR}\sim2\,\mathrm{ms}$$$.Conclusion
We have shown that the pronounced asymmetry in the bSSFP frequency response profile, stemming from intra-voxel frequency distributions, vanishes for $$$\mathit{TR}\rightarrow0$$$. At $$$3\,\mathrm{T}$$$, the asymmetry vanishes for $$$\mathit{TR}\sim1\,\mathrm{ms}$$$, whereas at lower clinical field strength, such as $$$1.5\,\mathrm{T}$$$, pure bSSFP imaging is expected for $$$\mathit{TR}\sim2\,\mathrm{ms}$$$ and thus becomes feasible for clinically relevant resolutions.Acknowledgements
This work was supported by the Swiss National Science Foundation (SNF grant No. 325230_182008).References
1. Carr H. (1958). Steady-state free precession in nuclear magnetic resonance. Physical Review 112(5): 1693
2. Miller K. L. (2010). Asymmetries of the balanced SSFP profile. Part I: theory and observation. Magnetic Resonance in Medicine, 63(2): 385-395.
3. Miller K. L., Smith S. M., Jezzard P. (2010). Asymmetries of the balanced SSFP profile. Part II: white matter. Magnetic Resonance in Medicine, 63(2): 396-406.
Figures