4190
Segmented 3D EPI with CAIPIRINHA for Fast, High-Resolution T2*-weighted Imaging1Siemens Healthcare, Brisbane, Australia, 2The University of Queensland, Brisbane, Australia, 3Cedars-Sinai Medical Center, Los Angeles, CA, United States, 4Siemens Medical Solutions USA, Baltimore, MD, United States, 5Siemens Medical Solutions USA, Baltimore, OH, United States, 6National Institute of Mental Health, Bethesda, MD, United States, 7Siemens Medical Solutions USA, Los Angeles, CA, United States, 8Siemens Healthcare, Sydney, Australia
Synopsis
In this work, a highly accelerated 3D EPI prototype sequence implementation and scanner in-line reconstruction are introduced. The proposed sequence provides a flexible combination of single-/multi-shot schemes, in-plane segmentation, image resolution, echo-train length, partial Fourier factors, 2D-CAIPIRINHA/2D-GRAPPA-based acceleration, and CAIPIRINHA shift. Initial tests presented herein indicate suitability for highly accelerated high-resolution susceptibility-based imaging with significantly reduced scan time, such as whole-head coverage at 0.65 mm isotropic resolution within 2 minutes.
Introduction
Susceptibility contrast due to T2* signal loss and phase accumulation from gradient echoes is essential for investigating neuronal activation1, parenchymal veins2, haemorrhages3, calcifications4, iron concentration5 and myelin content6 of the human brain. The spoiled gradient echo (GRE) sequence7 has been the workhorse for generating images with susceptibility contrast; however, its application in high-resolution acquisitions is often limited by long scan times. Three-dimensional echo-planar imaging (3D EPI) substantially reduces acquisition time and increases SNR efficiency8. If sufficient SNR is available, 3D EPI can be further accelerated, either to obtain high-resolution structural susceptibility-weighted images by combining multi-shot 3D EPI9 and GRAPPA10 acceleration11,12, or to reduce the volume repetition time and improve temporal resolution by skipping read-out (RO) lines and partitions from the single-shot (per partition) variant as used in functional neuroimaging13,14,15. In this work, we introduce a robust implementation of a 2D-GRAPPA- and 2D-CAIPIRINHA-accelerated 3D EPI sequence and scanner in-line reconstruction that straddle both implementations enabling flexible adjustments for spatiotemporal resolution (i.e., resolution, in-plane segmentation, as well as number of shots, including single-shot).Methods
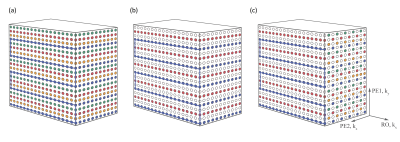
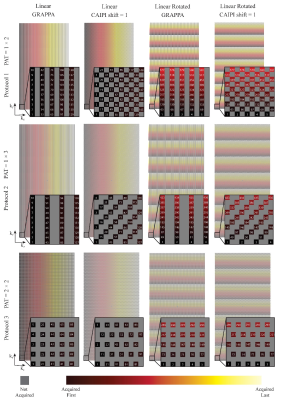
Figure 1 illustrates the k-space sampling pattern of a fully sampled multi-shot 3D EPI with 4 shots and 5 echoes per shot. As examples implemented in the current study, Figure 1(b) and (c) illustrate the 2-times accelerated k-space sampling with GRAPPA and CAIPIRINHA patterns, respectively. The GRAPPA acceleration was achieved by regularly skipping the 2nd and 4th shots for all partitions, whilst the CAIPIRINHA pattern was generated by alternating between skipping the 2nd and 4th shots and skipping the 1st and 3rd shots. Such under-sampling strategies can be extended to 2D-GRAPPA and 2D-CAIPIRINHA, as illustrated in Figure 2 with various acceleration factors. For completeness, the single-shot (per partition) protocol can be achieved by increasing the EPI factor so that EPI factor × Total Acceleration Factor = Base Resolution (number of RO lines per partition). In all cases, the 2D-GRAPPA and 2D-CAIPIRINHA patterns were designed to ensure continuation between segments without irregularities, and the central k-space line was sampled regardless of the sampling pattern employed. The 2D-CAIPIRINHA patterns generated by the proposed strategy herein do not require variable kz-gradient blips or variable echo spacing14, albeit producing similar 3D k-space sampling patterns. To test the performance of the proposed implementation, the sequence was run on two 3T scanners, a MAGNETOM Skyra and a MAGNETOM Prisma (Siemens Healthcare, Erlangen, Germany), using a 20Rx head/neck and a 64Rx head/neck coil, respectively. The 3D EPI protocols were configured in a sagittal orientation with whole-head coverage at 0.65 mm isotropic resolution. A 1-2-1 water excitation scheme was used with RF pulse TBW of 24 for all the scans. Integrated GRE pre-scans were used in the accelerated protocols to acquire auto-calibration data for image reconstruction. Detailed protocols are summarized in Figure 3, with the used acquisition patterns. In-line image reconstruction of both magnitude and phase images is available. Phase correction method “adaptive coil combination”16 was performed, which lead to phase images without the artefacts which affect phase images generated with conventional methods17.Results
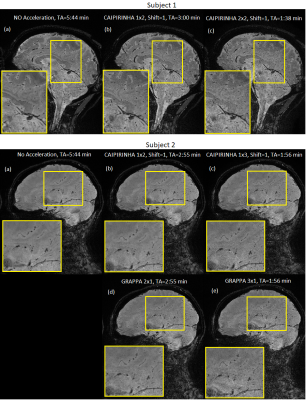
The in-line reconstructions are shown in Figure 4. With subject 1, the protocol was optimized for the contrast among white matter, grey matter and CSF, with a flip-angle of 10ᵒ. A flip-angle of 16ᵒ was employed for subject 2 to maximize grey matter SNR based on automated Ernst angle calculation, which resulted in reduced contrast. GRAPPA and CAIPIRINHA acceleration significantly reduced the acquisition time (TA) from 5:44 min with no acceleration to 2:55, 1:56, and 1:38 min with 2-, 3- and 4-times acceleration, respectively. Among the two subjects, accelerated acquisition presented no visually discernable motion- or acceleration-related artifacts. With 4 times acceleration, subject 1-(c) with a TA=1:38 min, image noise in cerebellum and brain stem regions became prominent, while the cortical SNR is mostly maintained. CAIPIRINHA 1x3 with shift=1, subject 2-(c) with a TA=1:56 min, provided an excellent balance between image quality and acceleration. In comparison with GRAPPA 3x1 acquisition, subject 2-(e), with identical total acceleration factor and TA, the CAIPIRINHA approach provided noticeably higher SNR.Discussion
The T2*-weighted imaging protocols as shown in Figure 3, may be further optimized, for example by enabling and adjusting the partial Fourier factor in the phase-encoding direction. This provides shortened TE and hence higher signal and reduced distortion; whilst the shortened TR may enable lower total acceleration factor for higher SNR with similar TA.The flexibility of the proposed sequence not only provides the high-spatial-resolution T2*-weighted scans as demonstrated, but also enables optimization on a continuous spatiotemporal-resolution spectrum. For example, initial testing suggests that whole-brain sub 2-second, 1.2 mm isotropic acquisition is feasible (Base Resolution=160, CAIPIRINHA acceleration=1x3, EPI factor=53, TE=30ms). In the future, even higher resolution for fMRI studies investigating depth-dependent and columnar signals might be achieved combining multi-shot with CAIPIRINHA acceleration18.
Conclusion
The proposed 3D EPI implementation enables a flexible combination of single-/multi-shot schemes, image resolution, in-plane segmentation, echo-train length, partial Fourier factors, CAIPIRINHA/GRAPPA-based acceleration, and CAIPIRINHA shift. The sequence was shown to be capable of producing whole-head coverage at 0.65 mm isotropic resolution within 2 minutes at 3T.Acknowledgements
S.B. acknowledges funding from the NHMRC-NIH BRAIN Initiative Collaborative Research Grant APP1117020, NIH grant 1R01MH111419.References
1. Ogawa S, Lee TM, Kay AR, Tank DW. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc Natl Acad Sci U S A. 1990;87(24):9868–72.
2. Reichenbach JR, Venkatesan R, Schillinger DJ, Kido DK, Haacke EM. Small vessels in the human brain: MR venography with deoxyhemoglobin as an intrinsic contrast agent. Radiology. 1997 Jul 1;204(1):272–7.
3. Scharf J, Bräuherr E, Forsting M, Sartor K. Significance of haemorrhagic lacunes on MRI in patients with hypertensive cerebrovascular disease and intracerebral haemorrhage. Neuroradiology. 1994 Oct;36(7):504–8.
4. Wu Z, Mittal S, Kish K, Yu Y, Hu J, Haacke EM. Identification of calcification with MRI using susceptibility-weighted imaging: a case study. J Magn Reson Imaging JMRI. 2009 Jan;29(1):177–82.
5. Haacke EM, Cheng NYC, House MJ, Liu Q, Neelavalli J, Ogg RJ, et al. Imaging iron stores in the brain using magnetic resonance imaging. Magn Reson Imaging. 2005 Jan;23(1):1–25.
6. Hwang D, Kim D-H, Du YP. In vivo multi-slice mapping of myelin water content using T2* decay. NeuroImage. 2010 Aug 1;52(1):198–204.
7. Haase A, Frahm J, Matthaei D, Hanicke W, Merboldt K-D. FLASH imaging. Rapid NMR imaging using low flip-angle pulses. J Magn Reson 1969. 1986 Apr 1;67(2):258–66.
8. Mansfield P, Maudsley AA, Bains T. Fast scan proton density imaging by NMR. J Phys [E]. 1976;9(4):271–8.
9. Zwanenburg JJM, Versluis MJ, Luijten PR, Petridou N. Fast high resolution whole brain T2* weighted imaging using echo planar imaging at 7T. NeuroImage. 2011 Jun 15;56(4):1902–7.
10. Griswold MA, Jakob PM, Heidemann RM, Nittka M, Jellus V, Wang J, et al. Generalized autocalibrating partially parallel acquisitions (GRAPPA). Magn Reson Med. 2002 Jun 1;47(6):1202–10.
11. Sati P, Thomasson DM, Li N, Pham DL, Biassou NM, Reich DS, et al. Rapid, high-resolution, whole-brain, susceptibility-based MRI of multiple sclerosis. Mult Scler J. 2014;20(11):1464–1470.
12. Sunil Patil, Henrik Odéen, John A. Derbyshire, Gunnar Krueger, Dennis L. Parker, Himanshu Bhat, et al. Accelerated Isotropic Sub-Millimeter Whole-Brain Susceptibility Imaging at 3T: Application to Multiple Sclerosis. In 2018. p. 5289.
13. Poser BA, Koopmans PJ, Witzel T, Wald LL, Barth M. Three dimensional echo-planar imaging at 7 Tesla. NeuroImage. 2010 May 15;51(1):261–6.
14. Narsude M, Gallichan D, van der Zwaag W, Gruetter R, Marques JP. Three-dimensional echo planar imaging with controlled aliasing: A sequence for high temporal resolution functional MRI. Magn Reson Med. 2016 Jun 1;75(6):2350–61.
15. Stirnberg R, Huijbers W, Brenner D, Poser BA, Breteler M, Stöcker T. Rapid whole-brain resting-state fMRI at 3 T: Efficiency-optimized three-dimensional EPI versus repetition time-matched simultaneous-multi-slice EPI. NeuroImage. 2017 Dec 1;163:81–92.
16. Jellus V, Kannengiesser ARS. Adaptive Coil Combination Using a Body Coil Scan as Phase Reference. Proc Intl Soc Mag Reson Med. 2014;22:4406.
17. Korbinian Eckstein, Siegfried Trattnig, Simon Daniel Robinson. An assessment of the ‘Prescan-Normalize Adaptive Combine’ approach to combining phase images from multi-channel coils at 3T. In: Proc ISMRM 2018. p. 4992.
18. Huber L, Finn ES, Chai Y, Goebel R, Stirnberg R, Stöcker T, et al. Layer-dependent functional connectivity methods. Prog Neurobiol. 2020 Jun 5;101835.
Figures