4187
A single-shot GRASE technique for rapid and distortion-free diffusion-weighted spinal imaging
Zhiqiang Li1, Melvyn B Ooi2, and John P Karis1
1Neuroradiology, Barrow Neurological Institute, Phoenix, AZ, United States, 2Philips Healthcare, Gainesville, FL, United States
1Neuroradiology, Barrow Neurological Institute, Phoenix, AZ, United States, 2Philips Healthcare, Gainesville, FL, United States
Synopsis
DWI plays a critical role in many neurological applications but its adoption in spinal imaging has been significantly hindered by geometric distortions in EPI, which is widely used in the clinic. TSE-based DWI methods, including PROPELLER and turboPROP, are free from distortion artifacts but may not be optimal for spinal imaging. In this work we present a single-shot GRASE technique with whole-mode acquisition and reduced FOV imaging to provide rapid and robust high-resolution spinal DWI. The feasibility is demonstrated and compared with ssEPI and msEPI in sagittal and axial cervical spine, and sagittal thoracic and lumbar spine.
Introduction
Spinal imaging comprises a significant portion of neurological examinations in clinical MRI. Diffusion-weight imaging (DWI) plays a critical role in revealing many neurological pathologies. However, its application in spinal imaging has not yet been widely adopted. The main challenge is the geometric distortion artifacts associated with single-shot echo-planar imaging (ssEPI), the most widely used DWI sequence. Various strategies have been studied to reduce the geometric distortion artifacts associated with EPI-based techniques. Parallel imaging, reduced field of view (FOV) methods1-3, and multi-shot acquisition4-6 have been implemented to reduce the echo train length and consequently the accumulated phase errors, which are the sources of the distortion artifacts. Nonetheless, residual artifacts are still problematic, especially when strong field variation exists. A distinct group of turbo-spin-echo-based (TSE) techniques7-9 are intrinsically insensitive to such distortion artifacts due to the refocusing RF pulses. However, a common disadvantage of these techniques is the low scan efficiency. Inspired by a recently enhanced turboPROP technique10, we propose a single-shot gradient- and spin-echo (ssGRASE) technique, with ZOOM (zonally magnified oblique multi-slice)1 and a whole-mode acquisition, for rapid and distortion-free high-resolution spinal DWI.Methods
Recent turboPROP developments show improved speed and robustness in brain DWI using a quadratic phase modulation scheme and a two-stage phase correction algorithm10. However, as turboPROP typically acquires a square FOV that covers the full anatomy within the slice, it is suboptimal for spinal imaging. To develop a technique suitable for spinal imaging, we propose a new technique with three strategies (Figure 1). First, a single-shot GRASE is implemented with Cartesian k-space sampling. This can be regarded as a special “single-blade” turboPROP, but enables a rectangular FOV that is optimal for spinal imaging. Second, a reduced FOV method, ZOOM1, is adopted to limit the phase FOV, which allows for high resolution in the phase encoding direction. Third, a whole-mode acquisition is proposed to further boost the resolution in the phase encoding direction by approximately a factor of 2 (compared to split-mode acquisition), without the need of parallel imaging and therefore without the g-factor penalty. Unlike the split-blade mode used in turboPROP, the whole-mode acquisition collects all odd and even echoes in the same k-space. Since odd and even echoes have different phases due to quadratic phase modulation, additional correction is required (Figure 2). In Fig. 2a, phase differences in gradient echoes are first corrected separately for odd and even echoes. In Fig. 2b, phase differences between odd and even echoes are then corrected using information derived from the odd and even SEs that are collected at the center of k-space. All these steps result in final k-space with phase differences corrected for across all echoes.The sequence was implemented on a Philips 3T Ingenia scanner. Volunteers were scanned in axial and sagittal planes of cervical spine, and sagittal plane of thoracic and lumbar spine. Sagittal imaging parameters include: thickness=3 mm, resolution=~2x1 mm2, one b=0 and three b=700 s/mm2 (800 for lumbar spine to better suppress CSF), echo train length=12, GRASE factor=5, 10 signal averages for b=700, FOV is trimmed to the specific segment. Axial C-spine is scanned with FOV=80x66 mm2, resolution=~1.1x1.1 mm2, thickness=5 mm. Scan times vary from 2:30~3:00. For reference, standard ssEPI and recently released product msEPI, all with ZOOM, were also collected with similar scan times and imaging parameters.
Results and Discussion
Figure 3 demonstrates the elimination of distortion artifacts with ssGRASE in sagittal C-spine. As indicated by the contour extracted from FLAIR images, the strong distortion artifacts in ZOOM-ssEPI render it not clinically preferred. These artifacts are dramatically reduced with ZOOM-msEPI; however, residual artifacts are still visible in the volunteer data acquired with optimized shimming on a well calibrated research scanner. It is expected that such artifacts are more prominent in the clinical settings. With the proposed ZOOM-ssGRASE technique, these artifacts are eliminated.Axial C-spine images are presented in Figure 4. Distortion artifacts in ssEPI in areas with strong field variation (superior and inferior slices) are reduced in msEPI, and eliminated in ssGRASE. Although the distortion artifacts in ssEPI and msEPI may not be visually disturbing in the middle slices of the axial plane, ssGRASE may provide better delineation of the nerve roots.
Figure 5 show examples of T- and L-spine. Similar to findings from C-spine images, the strong distortion artifacts in ssEPI are significantly reduced in msEPI with some residues, while eliminated in ssGRASE. The SNR of ssGRASE is comparable to that of msEPI. ssGRASE also provides better suppression of background artifacts and better delineation of the vertebral bodies, which is potentially more interesting for L-spine, where the vertebral bodies are the primary focus in many neurological applications (such as the claw sign for diagnosis of degeneration11). Slight shading in the inferior posterior corner of T-spine images with ssGRASE will be investigated.
Conclusion
In summary, an ssGRASE technique with reduced FOV, whole-mode acquisition, and corresponding enhanced phase correction has been developed for spinal imaging. ssGRASE provides better overall image quality with minimized distortion artifacts compared to ssEPI and msEPI, and comparable SNR to msEPI. Therefore, ssGRASE provides a potential alternative for robust clinical spinal DWI.Acknowledgements
No acknowledgement found.References
- Symms MR, Wheeler-Kingshott CA, Parker GJM, Barker GJ. Zonally-magnified oblique multislice (ZOOM) EPI. In Proceedings of the 8th ISMRM Annual Meeting, Denver, CO, USA. 2000:p160.
- Wilm BJ, Svensson J, Henning A, Pruessmann KP, Boesiger P, Kollias SS. Reduced field-of-view MRI using outer volume suppression for spinal cord diffusion imaging. Magn Reson Med. 2007;57:625-630.
- Saritas EU, Cunningham CH, Lee JH, Han ET, Nishimura DG. DWI of the spinal cord with reduced FOV single-shot EPI. Magn Reson Med. 2008;60:468-473.
- Holdsworth SJ, Skare S, Newbould RD, Guzmann R, Blevins NH, Bammer R. Readout-segmented EPI for rapid high resolution diffusion imaging at 3T. Eur J Radiol. 2008;65:36-46. PMCID: PMC3360876.
- Chen N-k, Guidon A, Chang H-C, Song AW. A robust multi-shot scan strategy for high-resolution diffusion weighted MRI enabled by multiplexed sensitivity-encoding (MUSE). Neuroimage. 2013;72:41-47. PMCID: PMC3602151.
- Jeong H-K, Gore JC, Anderson AW. High-resolution human diffusion tensor imaging using 2-D navigated multishot SENSE EPI at 7 T. Magn Reson Med. 2013;69:793-802. PMCID: PMC3424313
- Alsop DC. Phase insensitive preparation of single-shot RARE: Application to diffusion imaging in humans. Magn Reson Med. 1997;38:527-533.
- Schick F. SPLICE: Sub-second diffusion-sensitive MR imaging using a modified fast spin-echo acquisition mode. Magn Reson Med. 1997;38:638-644.
- Le Roux P. Non-CPMG Fast Spin Echo with Full Signal. J Magn Reson. 2002;155:278-292.
- Li Z, Ooi MB, Karis JP. An enhanced turboPROP+ technique for diffusion weighted imaging. In Proceedings of the ISMRM Virtual Conference and Exhibition, 2020:p966.
- Patel KB, Poplawski MM, Pawha PS, et al. Diffusion-weighted MRI “claw sign” improves differentiation of infectious from degeneative modic type 1 signal changes of the spine. AJNR Am J Neuroradiol. 2014;35:1647-1652.
Figures
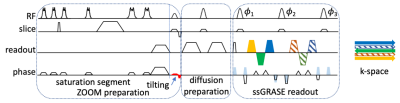
Fig. 1. Diagram of whole-mode GRASE. ZOOM preparation limits phase FOV. Following diffusion preparation is a single-shot GRASE readout with quadratic phase modulation. Odd (solid green) and even (stripped dark green) SEs are collected at the center of k-space, also serving as calibration data (even SEs are not visible in k-space due to overlapping with odd SEs). Odd and even GREs (solid and stripped blocks, respectively) are placed in an interleaving fashion in outer k-space. The colors represent different phases due to quadratic phase modulation and differences between GRE and SE.
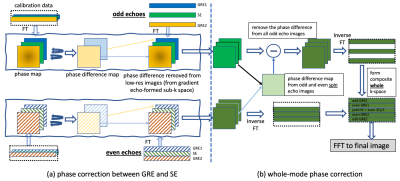
Fig. 2. Recon pipeline of whole-mode ssGRASE. Odd and even GRE-formed sub-k-space data are first corrected for phase errors separately (a). (b) shows additional phase correction for whole-mode acquisition. The phase difference maps between odd and even SE images are determined, which is possible because the odd and even SE are both collected at the center of k-space. These phase differences are removed from all odd echoes. Following inverse FT on all low-res odd/even GRE/SE images, those data are recombined to generate the final full k-space.
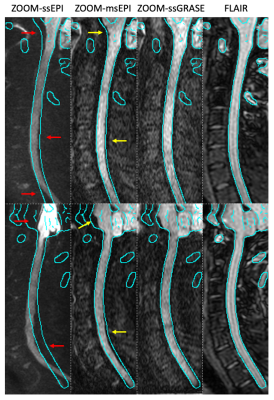
Fig. 3. Comparison of geometric distortion artifacts in 2 volunteers. The strong distortion artifacts in ssEPI (red arrows) are reduced but still present in msEPI (yellow arrows), while are eliminated in ssGRASE, as indicated by the contour extracted from FLAIR.
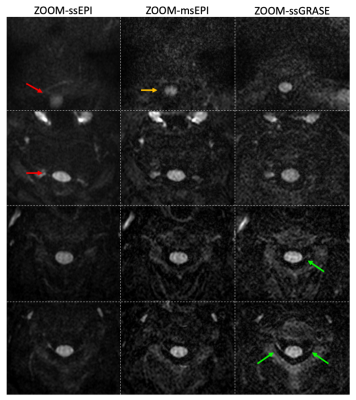
Fig. 4. Comparison of axial C-spine images from 2 volunteers. Th distortion artifacts in ssEPI in superior and inferior slices (red arrows) are reduced but still visible in msEPI (orange arrow), while are eliminated in ssGRASE. ssGRASE may also better delineate the nerve roots (green arrows).

Fig. 5. Illustration of T and L spine images. As can be observed, strong distortions exist in ssEPI. These artifacts are significantly reduced in msEPI with some residuals. msEPI may exhibit some artifacts (cyan arrows) and degraded quality of the vertebral body (orange arrows), which are improved in ssGRASE. It is typical that the spinal nerve bundle is not well seen in L-spine, where instead the vertebral bodies are the primary focus in many neurological applications. The shading in the lower corner of ssGRASE T-spine image (blue arrow) will be investigated.