4185
Accelerated Spiral Turbo-Spin-Echo Sequence with Split Spiral In-out Acquisition1Mayo Clinic, Rochester, MN, United States, 2Philips Healthcare, Rochester, MN, United States
Synopsis
Cartesian Turbo-spin-echo (TSE) has been widely used in routine clinical MRI but is subject to high specific absorption rate (SAR) and altered contrast due to long RF pulse train and reduced RF refocusing flip angles. As an alternative, spiral-TSE has been developed to produce low-SAR and improved contrast for fast T2-weighted imaging. In this work, we accelerate a previous spiral-TSE method by a factor of 2 using a split spiral-inout acquisition strategy with reversed arm orderings to intrinsically compensate for the T2-decay induced artifacts. In vivo brain experiment has been performed to demonstrate the feasibility of the proposed acquisition scheme.
Introduction
Turbo-spin-echo (TSE) sequences have been widely used in a range of clinical applications [1,2]. However, conventional Cartesian TSE is subject to high specific absorption rate (SAR) and altered contrast due to the long RF pulse train and reduced RF refocusing flip angles [3,4]. Alternatively, spiral TSE has been demonstrated [5] useful in producing low SAR and improved contrast for fast T2-weighted imaging owing to its high-SNR efficiency. In this study, we further accelerate spiral TSE by a factor of 2 using a split spiral in-out acquisition strategy.Method
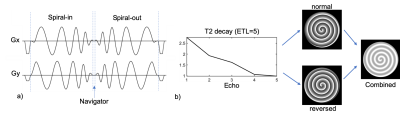
A spiral in-out trajectory is used in TSE sequence to achieve high acquisition efficiency (Fig. 1a). Previous work [5] required a double-encoding strategy (Fig. 1b) to compensate for the T2-decay induced signal variation during the echo train. Specifically, the second measurement reversed the order of the spiral arms obtained in the first measurement such that the average of the two measurements has reduced T2-decay induced artifact. In this study, we propose to split the spiral-in and spiral-out trajectory such that the spiral-out trajectory reverses the arm order of the spiral-in trajectory. Consequently, the eventual reconstruction from spiral-in and spiral-out will have similar reduced artifacts due to the average effect of spiral-in and spiral-out acquisition. A small delay between the spiral in and out trajectories produced data at k=0 which were used to correct for the average T2 signal loss across each arm. This non-uniform weighting was applied near the center of k-space, where signal energy is high and noise is low, to mitigate artifacts, however was quickly tapered to a uniform weighting throughout the rest of k-space, where signal energy is lower, to mitigate SNR losses from non-uniform data weighting.Experiment
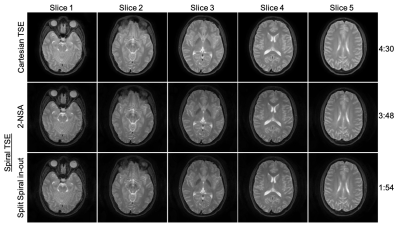
In vivo brain experiments were performed on a 3T MR scanner with the approval of institutional IRB and written informed consent from healthy subjects. Data were acquired using the spiral-TSE sequence with the previous double-encoding (2-NSA) and the proposed split spiral in-out strategies respectively (FOV = 230$$$\times$$$230 $$$mm^2$$$, resolution 0.65$$$\times$$$0.65 $$$mm^2$$$, 28 slices, slice thickness = 4 mm, RF refocusing flip angle = 180°, ETL = 5, TE shift 0 and 1.15 ms, 45 spiral arms, effective TE = 80 ms, TR = 3000 ms, total spiral in-out duration = 17.37 ms), taking approximately 3 min 48 sec and 1 min 54 sec respectively. Conventional Cartesian TSE with mDixon (TE shift 0 and 1.15 ms) was also carried out at the same FOV and resolution settings for comparison (refocusing flip angle = 120°, ETL = 16, effective TE = 80 ms, TR = 3000 ms, scan time = 4 min 30 sec). After proper gridding and Fast Fourier transform, the spiral images were processed using a joint water-fat separate and deblurring algorithm [6] to generate the target water images. The field inhomogeneity map was obtained separately from a low-resolution pre-scan. The spiral-in and spiral-out data were deblurred separately and then combined together.Results and Discussion
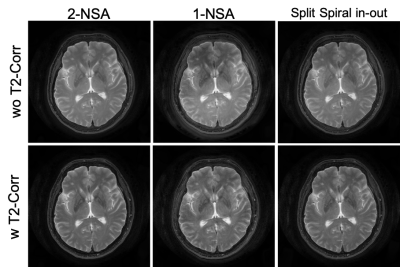
Brain images reconstructed from different spiral-TSE sequences were shown in Fig. 2. Spiral images reconstructed using only the first measurement (1-NSA) from the 2-NSA acquisition exhibit noticeable artifacts due to the T2-decay across the echo train. The proposed split spiral in-out method produces images with reduced artifacts comparable to the previous 2-NSA method. Comparison between Cartesian-TSE and spiral-TSE were shown in Fig. 3. In general, spiral-TSE was able to produce high-quality T2-weighted images compared to the conventional Cartesian-TSE method with low SAR (i.e., shorter ETL) and improved contrast (i.e., normal refocusing flip angle). Our proposed split spiral in-out method used only half of the scan time of the previous spiral-TSE method while maintaining similar artifacts reduction.Conclusion
An accelerated 2D spiral TSE technique is proposed and demonstrated considerable potential for fast T2-weighted imaging in the brain. Future work will include study advanced arm ordering and reconstruction to further reduce the T2-decay induced artifacts.Acknowledgements
This work is supported in part by a research funding from Philips Healthcare.References
[1] Mugler JP III. Optimized three-dimensional fast-spin-echo MRI. J Magn Reson Imaging. 2014;39:745-767.
[2] Busse RF, Hariharan H, Vu A, Brittain JH. Fast spin echo sequences with very long echo trains: design of variable refocusing flip angle schedules and generation of clinical T2 contrast. Magn Reson Med. 2006;55:1030-1037.
[3] Hennig J. Multiecho imaging sequences with low refocusing flip angles. J Magn Reson. 1988;79:397-407.
[4] Constable RT, Anderson AW, Zhong J, Gore JC. Factors influencing contrast in fast spin-echo MR imaging. Magn Reson Imaging. 1992;10:497-511.
[5] Li Z, Karis JP, Pipe JG. A 2D spiral turbo-spin-echo technique. Magn Reson Med. 2018 Nov;80(5):1989-1996
[6] Wang D, Zwart NR, Pipe JG. Joint water-fat separation and deblurring for spiral imaging. Magn Reson Med. 2018;79:3218- 3228.
Figures