4178
Simultaneous T1, T2 and T2* Mapping of the Carotid Plaque Using Combined Single- and Multi-echo 3D Golden Angle Radial Acquisition1Center for Biomedical Imaging Research, Department of Biomedical Engineering, School of Medicine, Tsinghua University, Beijing, China, 2Philips Healthcare, Beijing, China, 3School of Biomedical Engineering and Imaging Sciences, King's College London, London, United Kingdom, 4Department of Medicine, Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, MA, United States
Synopsis
Previous quantitative techniques for carotid plaque T1 mapping or T1, T2 mapping have been achieved using only one scan. Besides T1 and T2 quantification, T2* mapping of the carotid plaque is also important for the detection of iron deposition, which plays an important role in plaque progression. In this study, a new quantitative technique using combined single- and multi-echo 3D golden angle radial acquisition has been proposed for simultaneous T1, T2 and T2* mapping of the carotid plaque. The quantitative accuracy and the in-vivo feasibility of the proposed sequence have been demonstrated in phantom and volunteer studies.
Introduction
Quantitative techniques of the carotid atherosclerotic plaque have been drawing more and more attention1-6 as they evaluated the intrinsic tissue properties and had less variation between scans, while most techniques depended on multiple scans even for one parameter which may lead to misregistration between different sequences and increase the total scan time. To deal with these challenges, GOAL-SNAP sequence5 and SIMPLE sequence7 have been proposed to quantify T1 mapping or T1, T2 mapping of the carotid plaque in a single scan. In addition to T1 and T2 quantification, iron deposition, which can be quantified by T2* mapping, has also been reported to play an important role in plaque instability and progression4,8. Thus, in this study, we aim to develop a new sequence to quantify carotid plaque T1, T2 and T2* mapping simultaneously in a single scan using combined single- and multi-echo three-dimensional golden angle radial acquisition.Methods
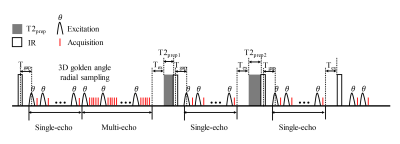
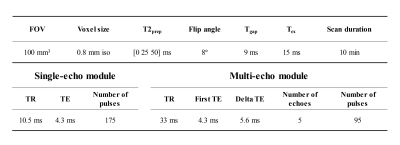
Sequence design: The proposed sequence extended the SIMPLE7 sequence (Figure 1). 3D golden angle radial single-echoes were acquired continuously after T2IR preparation and used for T1 and T2 quantification. Only after the IR without T2 preparation, golden angle radial multi-echoes were obtained after the single-echo acquisition to generate multiple T2* contrast and used for T2* quantification. Radial spokes acquired at the same inversion time (TI), same T2 preparation and same echo time (TE) were designed to satisfy the golden angle strategy9. Fat suppression was achieved using water excitation pulse. The scan parameters of the proposed sequence were shown in Table 1. Three T2 preparations (T2prep) of 0, 25, 50 ms were circulated. The total scan time of the proposed sequence was 10 min.Image Reconstruction and T1, T2, T2* fitting: Sliding window reconstruction with a temporal width of 25 spokes (262.5 ms/frame) was applied for the single-echo module, while for the multi-echo module, k-space data acquired from the same TE were combined to generate T2*-weighted images. Low-rank and sparsity constraint (LRS) reconstruction method10 was then performed on both of the single-echo and multi-echo modules to further improve the image quality and reduce the undersampling artifacts. The vessel wall image was reconstructed from the single-echo module using the data obtained around the nulling point of blood signal. T1 and T2 were estimated from the single-echo module by combining two extra 3D radial SPGR images using Bloch equation as in the SIMPLE sequence7. A mono-exponential decay curve was fitted to estimate T2* from the multi-echo module.
Phantom and in-vivo study: All scans were performed on a 3.0 T MR scanner (Ingenia CX, Philips Healthcare, Best, Netherlands). Phantom study was performed on 10 tubes filled with different concentrations of agarose and gadolinium-diethylenetriamine pentaacetic acid with different T1, T2 and T2* values. The T1, T2 and T2* quantification of the phantom using the proposed sequence were compared with traditional 2D inversion recovery spin echo (IR-SE), multi-echo spin echo (ME-SE) and multi-echo gradient echo (ME-GRE) sequences using Pearson correlation. After institutional review board approval and obtaining informed consent, 5 healthy volunteers (2 males, age 25±3 years) were scanned using a dedicated 8-channel carotid coil. In healthy volunteers, the proposed sequence was compared with 2D modified Look-Locker inversion recovery (MOLLI), multi-echo turbo spin echo (ME-TSE), and multi-echo turbo field echo (ME-TFE) in T1, T2 and T2* measurements. One elliptical ROI (64 mm2) was drawn within the muscle region and Wilcoxon rank-sum test was used for comparison.
Results
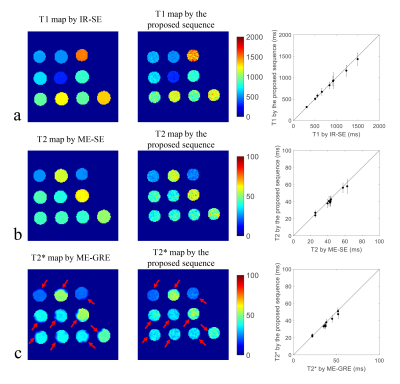
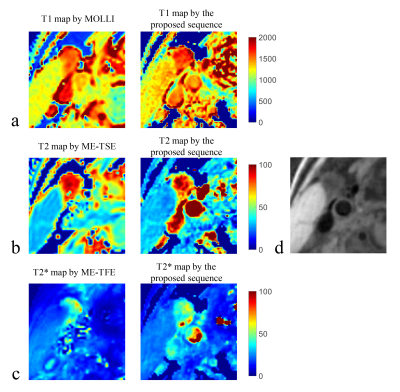
T1, T2 and T2* maps of the phantom estimated by the gold standard sequences and the proposed sequence were shown in Figure 2. In particular, the proposed sequence showed a similar T2*-shortening distribution with ME-GRE at the junction of the tube and the surrounding air (Figure 2c, red arrows). Thus for the following comparison of the mean values within the tubes, only the uniform central regions were averaged for T2* comparison. The mean T1, T2 and T2* values within each tube estimated by the proposed sequence showed good agreements with IR-SE (Pearson correlation coefficient (r)=0.996, p<0.001), ME-SE (r=0.993, p<0.001) and ME-GRE (r=0.989, p<0.001), respectively. The quantitative mapping results of one healthy volunteer (male, 30 years) were shown in Figure 3. The T1 of the muscle from all five volunteers calculated by the proposed sequence was higher than that by MOLLI (1219.9±51.1 ms vs. 1114.8±70.4 ms, p = 0.03). The T2 and T2* of the muscle estimated by the proposed sequence showed no statistically significant differences with ME-TSE (30.7±2.2 ms vs. 31.2±0.6 ms, p = 0.55) and ME-TFE (23.5±1.5 ms vs. 24.3±1.5 ms, p = 0.31), respectively. The proposed sequence also showed clear delineation of the vessel wall in the healthy volunteers (Figure 3d).Discussion and Conclusion
Quantitative T1, T2 and T2* measurements were achieved within one scan using the proposed sequence which combined the single- and multi-echo 3D golden angle radial acquisition. Good T1, T2 and T2* mapping agreements between the proposed sequence and the gold standard sequences were validated in the phantom study. The in-vivo feasibility of the proposed sequence has been demonstrated by the pilot volunteer study. In the future, more in-vivo studies on patients with carotid atherosclerotic plaque are needed to further investigate the clinical feasibility of the proposed sequence.Acknowledgements
None.References
1. Coolen BF, Heijtel DF, Potters WV, Nederveen AJ. 3D carotid wall T1 quantification using variable flip angle 3D merge with steady-state recovery. Paper presented at: In Proceedings of the 21st Annual Meeting of ISMRM 2013; Salt Lake City, Utah, USA.
2. Chai JT, Biasiolli L, Li LQ, et al. Quantification of Lipid-Rich Core in Carotid Atherosclerosis Using Magnetic Resonance T-2 Mapping Relation to Clinical Presentation. Jacc-Cardiovasc Imag. 2017;10(7):747-756.
3. Coolen BF, Poot DH, Liem MI, et al. Three-dimensional quantitative T1 and T2 mapping of the carotid artery: Sequence design and in vivo feasibility. Magnetic resonance in medicine. 2016;75(3):1008-1017.
4. Raman SV, Winner MW, 3rd, Tran T, et al. In vivo atherosclerotic plaque characterization using magnetic susceptibility distinguishes symptom-producing plaques. JACC Cardiovascular imaging. 2008;1(1):49-57.
5. Qi H, Sun J, Qiao H, et al. Carotid Intraplaque Hemorrhage Imaging with Quantitative Vessel Wall T1 Mapping: Technical Development and Initial Experience. Radiology. 2018;287(1):276-284.
6. G. Mihai SG, T. P. Sharkey-Toppen, S. V. Raman, S. Rajagopalan, O. P. Simonetti. Quantitative T1, T2 and T2* Mapping of Carotid Artery Normal Wall and Atherosclerotic Plaque. Proc Intl Soc Mag Reson Med 19. 2011;3311.
7. Qi H, Sun J, Qiao H, et al. Simultaneous T(1) and T(2) mapping of the carotid plaque (SIMPLE) with T(2) and inversion recovery prepared 3D radial imaging. Magnetic resonance in medicine. 2018;80(6):2598-2608.
8. Winner MW, 3rd, Sharkey-Toppen T, Zhang X, et al. Iron and noncontrast magnetic resonance T2* as a marker of intraplaque iron in human atherosclerosis. Journal of vascular surgery. 2015;61(6):1556-1564.
9. Chan RW, Ramsay EA, Cunningham CH, Plewes DB. Temporal stability of adaptive 3D radial MRI using multidimensional golden means. Magnetic resonance in medicine. 2009;61(2):354-363.
10. Qi H, Qiao H, Sun A, et al. Highly Undersampled Kooshball Reconstruction with Low-rank Modeling and Sparsity Constraints for High-resolution T1 Mapping. Paper presented at: In Proceedings of the 26th Annual Meeting of ISMRM 2018; Paris, France.
Figures