4174
Quantifying lactate using double quantum filtered 1H MRS with adiabatic refocusing pulses at 7 T1High Field MR Center, Center for Medical Physics and Biomedical Engineering, Medical University Vienna, Vienna, Austria, 2Department of Musculoskeletal & Ageing Science, Institute of Life Course and Medical Sciences, University of Liverpool, Liverpool, United Kingdom
Synopsis
The purpose of this study was to develop a novel single-shot acquisition scheme to quantify the lactate doublet at 1.3 ppm in skeletal muscle at ultrahigh field of 7 T. A double quantum filtered 1H MRS sequence was implemented using a Shinnar-Le-Roux optimised 90° pulse and adiabatic full-passage 180° pulses for slice-selective excitation and refocusing, respectively. The lactate resonance was successfully quantified in phantom (free lactate in solution) and ex vivo measurements (lactate injected into a meat specimen). Muscle fibre orientation and corresponding effects on the observed lactate resonance were in good agreement with the literature.
Introduction
Magnetic resonance spectroscopy offers non-invasive quantitative insights into the energy metabolism of skeletal muscle1. During moderate exercise the kinetics of phosphocreatine (PCr) measured by 31P MRS reflects oxidative capacity to resynthesise ATP2. However, at higher exercise intensities anaerobic glycolytic ATP synthesis makes a large contribution to the metabolic economy, which cannot be directly assessed with 31P MRS only. Usefully, lactate produced by reduction of glycolytically-generated pyruvate is directly related to ATP synthesis1, and lactate can be accessed using 1H MRS; however, its quantification in skeletal muscle is particularly challenging, even at long TE3, as large lipid signals overlap with the main lactate resonance, the methyl group doublet at 1.3 ppm. Additionally, orientation-dependent dipolar coupling effects4 significantly alter the apparent scalar modulation constant. These obstacles can be overcome using localised double quantum filtered (DQF) 1H MRS5,6. Although considered as the preferred method for unambiguous lactate quantification7, its efficiency at 7 T is limited by the bandwidth achievable with classical hard refocussing pulses. Frequency selective refocussing avoids this8, however at the cost of giving up 3D localisation. Here, we present a 3D-localised DQF 1H MR sequence using adiabatic refocusing pulses to unambiguously detect the lactate resonance at 1.3 ppm at 7 T.Methods
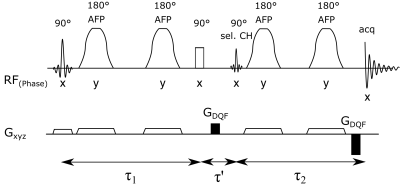
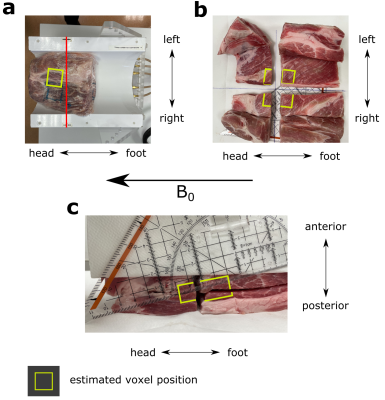
The pulse sequence consists of a slice-selective 90° excitation pulse (Shinnar-Le-Roux, 2.6 ms) followed by two pairs of adiabatic full-passage (AFP) 180° pulses (smoothed chirp, 4.6 ms) for perpendicular slice-selective refocusing (Figure 1). A non-selective 90° pulse (hard pulse, 0.5 ms) followed by a frequency-selective 90° pulse (Gaussian, 7.5 ms, BW = 120 Hz at 4.11 ppm), placed between the first and second pair of AFP pulses, creates the double quantum coherence which is then selected using spoiling gradients (ratio 1:2) and phase cycling. The performance of the sequence to detect the lactate resonance at 1.3 ppm was tested using a spherical phantom filled with lithium lactate and sodium acetate (diameter 160 mm, 96 mM and 100 mM) and in a pork specimen injected with sodium lactate (60 %), to investigate fibre orientation-dependent effects, using 4 different angles by turning the coil containing the specimen (0°–45°) relative to the B0 direction. The excitation frequency was set to –3.9 ppm relative to water, to reduce chemical shift displacement errors and spectra were acquired with a TR = 3 s and 30 averages, (VOIsphere = 27 ml, VOImeat = 17.1 ml). All measurements were performed on a Siemens dot Plus 7T scanner (Siemens, Erlangen, Germany) using either a dual-tuned 1H/31P loop coil (RAPID Biomedical GmbH, Germany) or a custom 1H/31P multi-channel transceiver surface coil array9. After MR measurements, the meat specimen was cut to assess the muscle fibre orientation within the selected voxel.Results
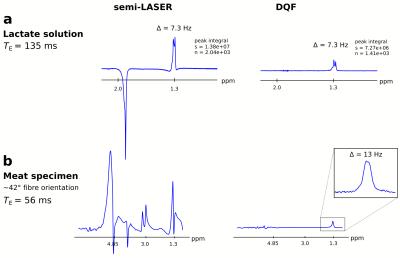
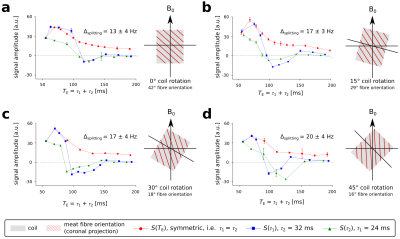
In solution, the lactate doublet at 1.3 ppm was observable with the DQF sequence, which achieved 53 % of the integrated signal area compared to the spectra acquired with conventional semi-LASER (both: TE = 135 ms, loop coil, see Figure 2a). The observed peak splitting of lactate in solution was 7.3 Hz, while the doublet acquired in meat showed 13 Hz splitting at ~42° fibre orientation (Figure 2a,b). Other resonances such as sodium acetate (~1.9 ppm, spherical phantom) or intramuscular fat (1–1.5 ppm, meat specimen) were near-perfectly suppressed by DQF. Varying the angle of the coil relative to the main magnetic field between 0° and 45° resulted in fibre angles between 42° and 16° (Figure 3), due to inclination of fibres in the sagittal direction. The effects of coil rotation on B1 were negligible. Splitting of the lactate doublet acquired from the meat specimen increased from 13 to 20 Hz (Figure 4). Modulations of TE and fibre angle resulted in the expected signal modulations, with faster modulation and phase inversion for echo times composed of either τ1 or τ2 held constant (Figure 4b-d), cf. Refs4–6.Discussion
The detection of the lactate doublet at 1.3 ppm, while suppressing unwanted resonances, was successfully achieved using the novel DQF acquisition scheme with adiabatic refocusing pulses at 7 T. The sequence performed best with a frequency offset of –3.9 ppm relative to water, fully reaching the theoretical efficacy of exploiting 50 % of the excitable magnetisation. Pulse durations were chosen to achieve the required 90° flip angles and full refocusing, while complying with the hardware constraints of the transmitter coil. The bandwidth of the adiabatic refocusing pulses was sufficient to maintain functionality of the DQF sequence (i.e. spectral bandwidth and minimising chemical shift displacement), which would be challenging or simply not feasible using conventional pulses (e.g. 3-lobe sinc) with similar pulse durations. Results from different orientations of the coil relative to the main magnetic field were in excellent agreement with the fibre orientation of the measured volume in the meat specimen. Overall results were in good agreement with literature, especially with the simulations in5,6 modelling signal evolution with different fibre orientations and echo time.Conclusion
We successfully demonstrated a novel acquisition scheme to quantify lactate using double quantum filtered 3D-localised 1H MR spectra with adiabatic refocusing pulses at 7 T. The dependence between the fibre orientation and the observable lactate signal amplitude underlines the importance of this phenomenon for future in vivo studies.Acknowledgements
No acknowledgement found.References
1. Kemp GJ, Ahmad RE, Nicolay K, et al. Quantification of skeletal muscle mitochondrial function by 31P magnetic resonance spectroscopy techniques: a quantitative review. Acta Physiol. 2015;213:107–144.
2. Chance B, Im J, Nioka S, et al. Skeletal muscle energetics with PNMR: personal views and historic perspectives. NMR Biomed. 2006;19:904-926. doi: 10.1002/nbm.1109.
3. Ren J, Sherry AD, Malloy CR. Noninvasive Monitoring of Lactate Dynamics in Human Forearm Muscle After Exhaustive Exercise by 1H-Magnetic Resonance Spectroscopy at 7 Tesla. Magn Reson Med 2013;70:610–619.
4. Asllani I, Shankland E, Pratum T, Kushmerick M. Anisotropic orientation of lactate in skeletal muscle observed by dipolar coupling in 1 HNMR spectroscopy. J Magn Reson 1999;139:213–224.
5. Asllani I, Shankland E, Pratum T, Kushmerick M. Double quantum filtered 1 H NMR spectroscopy enables quantification of lactate in muscle.J Magn Reson 2001;152:195–202.
6. Meyerspeer M, Kemp GJ, Mlynárik V et al. Direct noninvasive quantification of lactate and high energy phosphates simultaneously in exercising human skeletal muscle by localized magnetic resonance spectroscopy. Magn Reson Med. 2007;57:654–660.
7. Krššák M, Lindeboom L, Schrauwen-Hinderling V et al. Proton magnetic resonance spectroscopy in skeletal muscle: Experts’ consensus recommendations. NMR in Biomedicine. 2020:1–20.
8. Boer VO., Luijten PR, Klomp DWJ. Refocused Double-Quantum Editing for Lactate Detection at 7 T. Magn Reson Med 2013;69,1–6.
9. Goluch S, Kuehne A, Meyerspeer M, et al. A form-fitted three channel 31P, two channel 1H transceiver coil array for calf muscle studies at 7T. Magn Reson Med. 2015;73:2376-2389.
Figures