4172
Combined Echo Two-Point Dixon method for high efficiency water/fat separation1Siemens Shenzhen Magnetic Resonance Ltd., Shenzhen, China
Synopsis
Spin-echo-based Dixon sequences may suffer from reduced SNR efficiency, due to dead time necessary for the readout shifts. In this abstract, an imaging method utilizing two pairs of fast-switching bipolar readout gradients and partially-opposed-phase and in-phase Dixon (CETD) is proposed to further reduce dead time. More attractively, the novel steps in joint reconstruction of the two pairs ensure the consistency of water-fat separation.
Introduction
Dixon method is a model-based water suppression technique robust to B0 inhomogeneity. It deploys the chemical shift dephasing by shifting the readout from the spin echo. However, original spin echo-based Dixon sequence suffers from reduced SNR efficiency, due to dead time necessary for the readout shifts. Several Dixon methods have been proposed to improve its efficiency, including fast spin echo triple-echo Dixon [1] and generalized two-point Dixon method using partially opposed-phase and in-phase echoes [2]. In this abstract, an imaging method utilizing two pairs of fast-switching bipolar readout gradients and partially opposed-phase and in-phase Dixon is proposed to further reduce dead time. More attractively, the novel steps in joint reconstruction of the two pairs ensure the consistency of water-fat separation.Methods
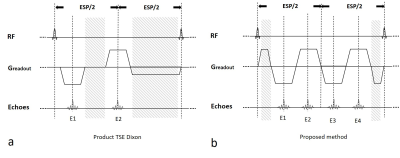
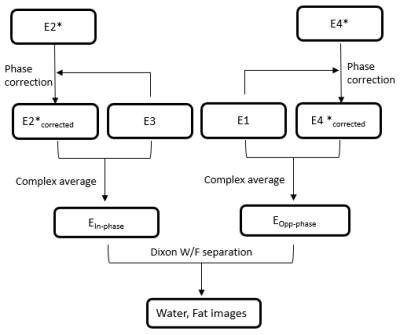
Figure 1a shows part of the diagram of TSE Dixon sequence where dead time ispresent. At low field, dead time is substantial as longer time is required to achieve opposed phase for the first echo, results in reduced sampling efficiency. The diagram of the proposed Combined Echo Two-points Dixon (CETD) method is shown in figure 1b. Four echoes (E1-4) are acquired in one echo spacing period. E2 and E3 close to the center, are partially in-phase, while E1 and E4 are partially opp-phase. Each pair of echoes, E1 and E3, E2 and E4, acquired with the same readout gradient polarity, so that water-fat separation can be performed without phase correction. The two pairs have the same dephasing factor but opposite gradient polarities. Note the high acquisition efficiency of this method, all the time between refocusing pulses are used for data sampling except the readout pre-phasing and re-phasing gradients.Echoes 1-4 undergo a joint Dixon reconstruction after regular reconstruction steps, as shown in figure 2. First 2D phase correction between the complex conjugate of the E2, i.e., E2* and E3 is performed. This is realized by subtracting the 2D low-passed phase difference between E2* and E3 from E2*. This phase difference is related with the difference of positive and negative readout gradients. Then the two in-phase images, E2*corrected and E3, are averaged for SNR boost as one Ein-phase. Eopp-phase is obtained likewise. Finally, Dixon water-fat separation is performed for the Ein-phase and Eopp-phase to output water and fat images.
The sequence and image reconstruction prototype were implemented using IDEA (Integrated development environment for application, Siemens). The experiments were performed at Siemens MAGNETOM 0.55T scanner.
Results
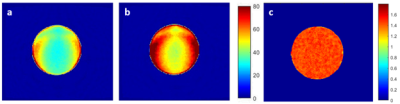
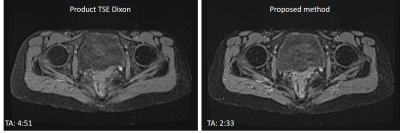
Figure 3 shows SNR maps of phantom images acquired with the same acquisition time using product TSE Dixon (a), that with our Combined Echo Two-points Dixon (CETD) (b), and the ratio between CETD and product (c). SNR was calculated using a pseudo multiple replica method [3]. The CETD method enabled 30% - 40% SNR improvement. Note that the CETD resulted in large dephasing factor, 0.98 comparing to 0.67 of conventional fast TSE Dixon.In vivo T1 weighted Dixon data were also collected for hip in one healthy volunteer. In figure 4, a comparison of the water images acquired with TSE Dixon and CETD shows that the latter provided comparable image quality with a much shorter scan time (4 min 51s comparing to 2 min 33s). Other parameters used in the product sequence were TE/TR = 17 / 627 ms, average = 2, dephasing factor = 0.67, and in the proposed method: TE/TR = 18 / 671 ms, average = 1, dephasing factor = 0.98. Same geometric parameters were used in the two protocols: resolution: 1.1 x 1.1 x 3.5 mm3, number of slices: 25, bandwidth: 406Hz/Pixel.
Discussions
In this study, we implemented a novel two-points Dixon technique with combined echoes and demonstrated that it significantly boosted SNR compared to conventional TSE Dixon since the acquired echoes were doubled within the very similar acquisition time. This high sampling efficiency was achieved because almost all the time between two refocusing pulses were used for data acquisition, except the time occupied by readout pre-phasing and re-phasing gradients. There was also novelty in image reconstruction steps: while it was possible to process the four echoes in completely two separate pairs, we implemented a phase correction process before averaging to reduce redundancy and ensured the consistent water-fat separation results, both locally and globally. Future study can incorporate asymmetric echo technique to increase the flexibility of choice of TEs of echoes. Also, the choice of TEs of E2/E3 and E1/E4 are flexible as long as the water-fat separation is robust and noise amplification is acceptable. For example, at the field strength below 1.5T, E2/E3 and E1/E4 are set to close to in-phase and out-of-phase, respectively. While at 3T they can be set in another way, i.e., E2/E3 closing to out-of-phase and E1/E4 closing to in-phase.Conclusions
The proposed combined echo two-points Dixon provides an efficient Dixon imaging method. It incorporates the fast-switching bipolar readout gradients for partially opposed-phase and in-phase echoes acquisition to effectively reduce dead time and boost SNR and a joint reconstruction of the two pairs to ensure the consistency of water-fat separation.Acknowledgements
No acknowledgement found.References
1. Son JB, Hwang KP, Madewell JE, et al. A flexible fast spin echo triple-echo Dixon technique. Magn Reson Med. 2017;77(3):1049‐1057.
2. Eggers H, Brendel B, Duijndam A, Herigault G. Dual-echo Dixon imaging with flexible choice of echo times. Magn Reson Med. 2011;65(1):96‐107.
3. Robson PM, Grant AK, Madhuranthakam AJ et al. Comprehensive Quantification of Signal-to-Noise Ratio and g-Factor for Image-Based and k-Space-Based Parallel Imaging Reconstructions. Magn Reson Med. 2008; 60(4):895-907
Figures