4168
Improved echo-split GRASE imaging: a single-shot parametric T2 mapping protocol with removal of contamination from multiple echo pathways
Mei-Lan Chu1, Tzu-Cheng Chao2, Nan-kuei Chen3, and Hsiao-Wen Chung1
1Graduate Institute of Biomedical Electronics and Bioinformatics, National Taiwan University, Taipei, Taiwan, 2Mayo Clinic, Rochester, MN, United States, 3University of Arizona, Tucson, AZ, United States
1Graduate Institute of Biomedical Electronics and Bioinformatics, National Taiwan University, Taipei, Taiwan, 2Mayo Clinic, Rochester, MN, United States, 3University of Arizona, Tucson, AZ, United States
Synopsis
Our novel single-shot T2 mapping framework, integrating ES-GRASE acquisition and parametric mapping, has the following advantages. First, signal contamination of high-order echoes is removed in the propose framework. Second, T2 relaxation times can be accurately measured by incorporating parallel imaging and multi-echo-pathway signal modeling into the reconstruction procedure.
Introduction
Conventional T2 mapping protocols are vulnerable to signal contamination from multiple echo pathways. To address this problem, here we propose an improved Echo-split GRASE (ES-GRASE) imaging technique1 which can eliminate all the unwanted echo pathway, while retain only primary spin-echo pathway for measuring T2 values. The proposed imaging sequence can be adaptable to existing reconstruction framework and provide a more reliable T2 measurement.Methods
Data acquisitionThe proposed ES-GRASE sequence is illustrated in Figure 1, in which the EPI readout train in each echo acquires 1/4 k-space data corresponding to different echo times (TEs). The prewinding and rewinding gradients are designed to dephase most of the high-order echo pathways. The different directions of refocusing RF pulses are designed to generate 90° phase difference between the primary and residual high-order pathways. Specifically, in the first and second echoes, only signal from the primary echo pathway (red arrows in Figure 1) is refocused on corresponding TEs. In the third and fourth echoes, the signal from the primary and high-order pathways (blue arrows in Figure 1) are orthogonally separated (i.e. 90° out-of-phase) in the transverse plane.
The out-of-phase property between the primary and high-order echo pathways is different when background phase is involved. The background phase $$$\theta$$$ exists in all pathways, while it is rotated to the opposite direction through the primary and high-order pathways in the third and fourth echo, as shown in Figure 1. The opposite rotation induces a 90+$$$\2theta$$$° out-of-phase between the pathways.
Data processing
To acquire the purely T2-decay signals from the primary echo pathway, we developed a data processing framework to exclude the high-order pathways in the third and fourth echoes as shown in Figure 2. Images of four echoes were reconstructed using parallel imaging techniques and the background phase $$$\theta$$$ was estimated. The purely T2-decay signals can be obtained by extracting the real part of the background-phase-removed signal of the third and fourth echoes.
T2 measurement
To measure the T2 value, we developed a signal model of the ES-GRASE sequence including inter-CPMG-echo information (pure T2-exponential decay), weighted information of the primary echo pathway, and flip angles (FAs) of RF pulses. The model is $$$M_0^{(k)}(r)=(sin^2\frac{\alpha}{2})^ke^{\frac{-TE_k}{T_2(r)}}M_0(r)$$$, in which $$$M_0^{(k)}(r)$$$ is the pure-T2-weighted image-domain signal intensity of the $$$k$$$-th echo at spatial location $$$r$$$. $$$(sin^2\frac{\alpha}{2})^k$$$ is an intensity-correction term for the $$$k$$$-th echo signal from the primary echo pathway, corresponding to the FA $$$\alpha$$$. $$$TE_k$$$ is the echo time for the $$$k$$$-th echo and $$$T_2(r)$$$ is the T2 time constant to be measured.
The T2 time constant can be estimated by exponentially fitting the signal from $$$M_0^{(1)}$$$ to $$$M_0^{(4)}$$$ if the FA $$$\alpha$$$ is known. In this project, we propose to use a modified four-shot spin-echo EPI sequence in which slice-selection and phase-encoding gradients are applied along the same direction to produce a high-quality FA measurement1.
Simulation study
The proposed framework was evaluated by computer simulation. The improved ES-GRASE and conventional GRASE imaging sequence were simulated based on Bloch equations and extended phase graph theory2,3. To assess the performance of T2 measurement, we performed a series of experiments with multiple FAs and background phases.
First, three sets of the single-shot ES-GRASE data with four echoes were acquired using a 8-channel coil with TE = 30, 60, 90, and 120 ms, matrix size = 128x128, and background phase = 6°. The FAs of the refocusing pulses are 120°, 150° and 180° respectively. Second, three sets of the conventional GRASE4 data with four echoes were acquired with the same imaging parameters as the previous three experiments. Noise are simulated by adding Gaussian white noise (zero mean and variance $$$10^{-5}$$$) to the proton density signal for all experiments. The complex-valued coil sensitivity maps were calculated based on simulations using Biot-Savart's law.
To reconstruct high-quality images, the N/2 ghost was removed using a phase cycling method5, and the down-sampled images were reconstructed with the SENSE method6. The T2 time constant is then calculated pixel-by-pixel by exponentially fitting the signal from the images.
Results
Figure 3 and 4 show the experimental results, in which the estimated T2 maps from the proposed technique and the conventional method are presented respectively. When FAs were 180°, the T2 values were accurately calculated by both methods since only the primary echo pathway was presented in the data. When FAs were not 180° (i.e. FAs = 120° and 150°), the T2 values were overestimated by the conventional method due to the higher signal intensity from the overlapped echo pathways. On the other hand, the proposed protocol reliably provided accurate T2 values when FAs were not 180°.Furthermore, smaller FA results in higher standard deviation (i.e. noise level) of the T2 value when using the proposed method. The higher noise level comes from lower signal intensity of the separated primary echo pathway corresponding to smaller FA.
Discussion and conclusions
Here we report a robust single-shot T2 mapping protocol enabled by improved ES-GRASE scan. Signal contamination from high-order echoes is removed in the developed framework, and T2 maps can be accurately measured. Experimental results demonstrate the successful performance of this new technology on improving the accuracy and scan efficiency of parametric T2 mapping.Acknowledgements
No acknowledgement found.References
- Chu, ML, MRM 2018; 79:383
- Hennig, J, MRM 1986; 3(6):823
- Hennig, J Concepts in MR 1991; 3
- Oshio, K MRM 1991; 20:344.
- Chen, N, MRM 2011;66(4):1057
- Pruessmann K.P. MRM 1999; 42(5):952
Figures
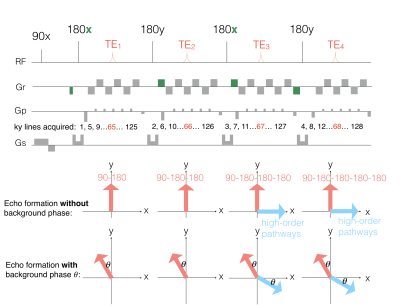
The single-shot ES-GRASE sequence. The areas and polarities of prewinding and rewinding gradients (i.e. the green gradient) of the readout gradient are designed to remove high-order echo pathways. The alternating direction of the refocusing RF pules (i.e. 180x-180y-180x-180y) can generate 90° phase difference between the primary and residual high-order echo pathways. The phase difference is 90+2𝜃° when the background phase is involved.
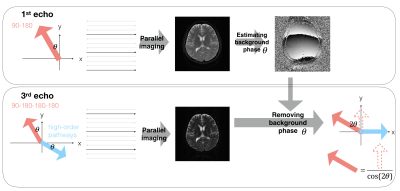
Data preprcessing frameowrk: First, images of four echoes were reconstructed using parallel imaging techniques, and the N/2 ghost was removed using phase cycling method. Second, the background phase was estimated from the first-echo image (see the upper panel). Third, rotating the signal from the third and fourth echo with the estimated background phase . The real part of the rotated signal is only from the signal of the primary echo pathway, with which the primary echo signal can be calculated by basic Trigonometry (see the lower panel in which only the third echo is shown as an example).
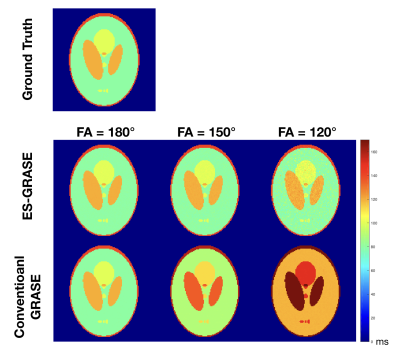
Experimental results: T2 maps from ground truth data, data acquired by ES-GRASE and conventional GRASE with three different FAs.
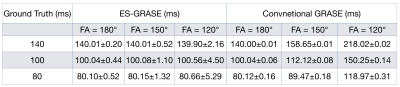
Experimental results: the mean and standard deviation of the T2 values within selected ROI from ground truth data, data acquired by ES-GRASE and conventional GRASE with three different FAs.