4163
Investigation into the Cerebrovascular Effects of Gender Affirming Therapy in Transgender Men using TOF-MRA and pCASL1Département de médecine nucléaire et radiobiologie, Université de Sherbrooke, Sherbrooke, QC, Canada, 2Département de pédiatrie, Université de Sherbrooke, Sherbrooke, QC, Canada, 3Département de radiologie diagnostique, Université de Sherbrooke, Sherbrooke, QC, Canada
Synopsis
We investigated if gender affirming hormonal therapy (GAHT) in young transgender men receiving testosterone (T) impacts cerebrovascular function and structure using pseudo-continuous arterial spin labelling (pCASL) and time of flight magnetic resonance angiography (TOF). We observed decreases in CBF and arterial vessel diameter. These results suggest an association between increased serum T and decreases in CBF which may be related to changes in cerebrovascular morphology.
Introduction
GAHT is a safe and effective option for transgender individuals. In the case of transgender men, the goal is to masculinize the body through the administration of T1. However, it is currently unknown if the masculinizing effects extend to cerebrovascular function and structure. There exists a well-established sex difference in CBF such that men have lower CBF than women2. Interestingly, this sex difference becomes particularly pronounced after puberty3 which suggests that sex hormones play an important role in the lower CBF observed in men. However, the exact role of T on CBF and cerebrovascular structure remains largely unclear, and if GAHT in transgender men will result in a masculinization of the brain such that CBF decreases, becoming similar to cisgender men. Therefore, the goal of this study was to investigate the effects of T on cerebrovascular function and structure in young transgender men receiving GAHT.Methods
Two young transgender men were referred by their endocrinologist prior to the start of GAHT. Upon giving informed, written consent, each participant completed an MRI protocol and blood test before starting GHAT and between 3 to 6 months into their GAHT. Participants were imaged on a 3T Insignia scanner equipped with a 32-channel head-coil (Philips Healthcare, Best, Neatherlands). First, a 3D gradient-echo T1 weighted image was collected (field of view (FOV)=240X240X161 mm; TR/TE=7.9/3.5ms, 1 mm isotropic voxels, flip angle(FA)= 8°), followed by a high resolution, whole-brain multi-band TOF sequence (FOV= 200X200X120.9mm, TR/TE= 23/3.45ms, FA= 18°, parallel imaging (SENSE) acceleration factor=3, acquisition resolution of 0.65X0.65X1.30, reconstructed resolution of 0.626x0.625x0.65mm) and a pCASL sequence (background suppression, label duration=1650ms, postlabel delay=1800ms, 2D multislice EPI readout, TR/TE 4246/16ms, 22 4mm, 3x3 resolution, FOV=240X240mm, effective temporal resolution 8492ms).Each participant’s TOF was registered to their T1 image from the pre-GAHT visit using ANTS4. The vascular tree was segmented from the TOF image using a method developed in our laboratory5 to create a 3D image of the cerebral vascular tree, and lumen diameters were estimated. CBF was estimated using Explore ASL6 and registered to their pre-GAHT visit T14.
In order to exam regional variations in CBF and its relationship to arterial diameters, the CBF maps from pre and post GAHT were subtracted. The average, whole-brain absolute change in CBF was computed and subtracted from the difference image to create a mask of the regions with a change in CBF above the average absolute change in CBF. This mask was used to compare the number of voxels making up the segmented vascular tree (TOF-voxels) near regions of large changes in CBF pre and post GAHT. Next, large arterial diameters within the Circle of Willis (COW) and smaller arterial diameters outside of the COW were sorted according to their diameter and compared pre and post GAHT. Changes in serum hormone, CBF, TOF-voxels, and arterial diameters are expressed as a percentage change from the pre-GAHT session.
Results
CBF decreased in both participants, whole-brain CBF maps pre and post GAHT are shown in Figures 1A and 1B. Participant 1’s CBF decreased by 11.12 % (Figure 1C, navy bar; Pre-GAHT CBF: 56.78±23.80 ml/100g/min; Post-GAHT CBF: 50.64±21.90 ml/100g/min) and participant 2’s CBF decreased by 25.06% (Figure 1C, green bar; Pre-GAHT CBF: 37.84±18.84 ml/100g/min; Post-GAHT CBF: 37.53±16.23 ml/100g/min ). The magnitude of the change in CBF appears to be related to the magnitude of change in T (Figure 1B). Segmented arterial trees are presented in Figure 2A and Figure 2B. Changes in the arteries of the COW were small and did not appear related to changes in T. However, we observed decreases in TOF-Voxels of -12.67% in participant 1 (navy bar in Figure 2B) and -24.61% in participant 2 (green bar in Figure 2C). The decrease in TOF-Voxels was of a similar magnitude as the change observed in the CBF suggesting these changes may be related to changes in T. Lastly, we found that number of voxels corresponding to small-diameter arteries decreased in all categories in both participants (Figure 2D).Discussion
Our results suggest that T decreases CBF and that this reduction may be driven by vasoconstriction of small arteries. Nevertheless, due to the well-documented effects of T on hematocrit (HcT)1,7–9 these effects may also be related to changes in the hematological profile of our participants which we cannot rule out with the current results. Notably, changes in HcT can influence blood viscosity10, which has an impact on the longitudinal relaxation time of the blood11 which can bias CBF quantification11,12. Of note, the reduction in TOF-Voxels suggests a decrease in the TOF signal in the small arteries of our participants. It is currently unclear what may be the driving force behind these changes. To address these confounds, in future participants we will measure HcT, and estimate T1-blood and arterial transit time.Conclusion
In conclusion, we have shown in young transgender men that GAHT is associated with decreases in whole-brain CBF and reduction in segmentation and diameter of small cerebral vessels with no change in the COW. These results suggest that GAHT masculinizes the brain resulting in a CBF pattern similar to cisgender men.Acknowledgements
We would like to thank the participants for volunteering their timeReferences
1. Wierckx K, Van Caenegem E, Schreiner T, et al. Cross-sex hormone therapy in trans persons is safe and effective at short-time follow-up: Results from the European network for the investigation of gender incongruence. Journal of Sexual Medicine. 2014;11(8):1999-2011. doi:10.1111/jsm.12571
2. Henriksen OM, Kruuse C, Olesen J, et al. Sources of variability of resting cerebral blood flow in healthy subjects: A study using 133 Xe SPECT measurements. Journal of Cerebral Blood Flow and Metabolism. 2013;33(5):787-792. doi:10.1038/jcbfm.2013.17
3. Kaczkurkin AN, Raznahan A, Satterthwaite TD. Sex differences in the developing brain: insights from multimodal neuroimaging. Neuropsychopharmacology. 2019;44(1):71-85. doi:10.1038/s41386-018-0111-z
4. Avants BB, Tustison N. Advanced normalization tools (ANTS). 2009.
5. Bernier M, Cunnane SC, Whittingstall K. The morphology of the human cerebrovascular system. Human Brain Mapping. 2018;39(12):4962-4975. doi:10.1002/hbm.24337
6. Mutsaerts HJMM, Petr J, Groot P, et al. ExploreASL: An image processing pipeline for multi-center ASL perfusion MRI studies. NeuroImage. 2020;219(June). doi:10.1016/j.neuroimage.2020.117031
7. Connelly PJ, Freel EM, Perry C, et al. Gender-affirming hormone therapy, vascular health and cardiovascular disease in transgender adults. Hypertension. 2019;74(6):1266-1274. doi:10.1161/HYPERTENSIONAHA.119.13080
8. Meyer G, Mayer M, Mondorf A, Flügel AK, Herrmann E, Bojunga J. Safety and rapid efficacy of guideline-based gender-affirming hormone therapy: an analysis of 388 individuals diagnosed with gender dysphoria. European Journal of Endocrinology. 2020;182(2):149-156. doi:10.1530/EJE-19-0463
9. Defreyne J, Vantomme B, Van Caenegem E, et al. Prospective evaluation of hematocrit in gender-affirming hormone treatment: results from European Network for the Investigation of Gender Incongruence. Andrology. 2018;6(3):446-454. doi:10.1111/andr.12485
10. Grotta J, Ackerman R, Correia J, Fallick G, Chang J. Whole blood viscosity parameters and cerebral blood flow. Stroke. 1982;13(3):296-301. doi:10.1161/01.STR.13.3.296
11. Hales PW, Kirkham FJ, Clark CA. A general model to calculate the spin-lattice (T1) relaxation time of blood, accounting for haematocrit, oxygen saturation and magnetic field strength. Journal of Cerebral Blood Flow and Metabolism. 2015;36(2):370-374. doi:10.1177/0271678X15605856
12. Wu W, St Lawrence KS, Licht ÞDJ, Wang DJJ. Quantification Issues in Arterial Spin Labeling Perfusion. Magnetic Resonance Imaging. 2011;21(2):65-73.
Figures
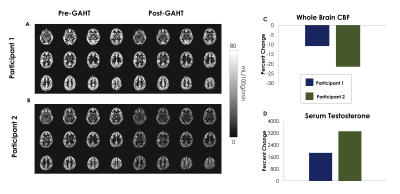
A) Whole-brain CBF pre and post GAHT treatment in participant 1.
B) Whole-brain CBF pre and post GAHT treatment in participant 2.
C) Percent change in average whole-brain CBF. Note that percent change in CBF appears to be of the same magnitude as the change in Serum T shown in D.
D) Percent change in Serum T.
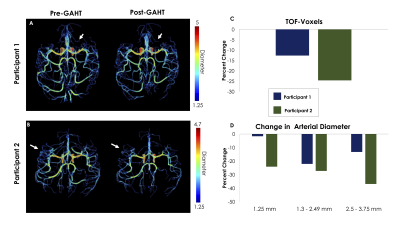
A) 3D projection of segmented vascular tree, with diameter values in participant 1. White arrows indicate regions where TOF-Voxels decreased after GAHT.
B) 3D projection of segmented vascular tree, with diameter values in participant 2. White arrows indicate regions where TOF-Voxels decreased after GAHT.
C) Percent change of TOF-Voxels. The decrease of TOF-Voxels is of the same magnitude as the decrease in CBF.
D) Change in arterial diameter in small arteries. Note that across all groups of small vessels there was a decrease in the number of voxels with in each diameter class.