4149
Integration of high-resolution ultra-high-field 7T magnetic resonance vessel neuroimaging into clinical routine: preliminary results1Institute of Diagnostic and Interventional Neuroradiology, Bern University Hospital, Inselspital,, Bern, Switzerland, 22. Advanced Clinical Imaging Technology, Siemens Healthcare AG, Bern, Switzerland, 33. Translational Imaging Center, Sitem-Insel, Bern, Switzerland, 44. Departments of Radiology and Biomedical Research, University of Bern, Bern, Switzerland, 55. Advanced Clinical Imaging Technology, Siemens Healthcare AG, Lausanne, Switzerland, 66. Department of Radiology, , Lausanne University Hospital and University of Lausanne, Lausanne, Switzerland, 77. LTS5, École Polytechnique Fédérale de Lausanne (EPFL), Lausanne, Switzerland, 86. Department of Radiology, Lausanne University Hospital and University of Lausanne, Lausanne, Switzerland
Synopsis
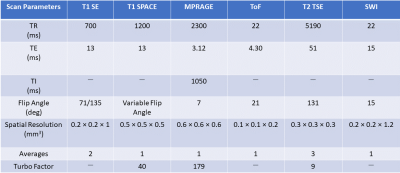
MRI-based vessel imaging has two goals, the anatomico-morphological depiction and vessel wall imaging, including delineation of contrast enhancement within the vessel wall. We performed sequence implementation and optimization in healthy volunteers to establish a dedicated 7T MRI protocol for vessel imaging at 7T. We implemented MPRAGE, T2 TSE, SWI, and ToF for anatomico-morphological vessel-delineation and optimized T1 SPACE and T1 SE for vessel wall delineation.
Introduction
Recently, ultra-high-field MR imaging at 7 Tesla has become available as a clinical non-invasive imaging modality. Due to the higher field strength, UHF 7T MRI enables non-invasive imaging with increased signal-to-noise ratio (SNR) resulting in improved spatial resolution and vessel contrast. Several studies suggested superiority of 7T imaging over 1.5T or 3T MRI in depiction and characterization of neurovascular pathologies [1]. Strong correlation between 7T and digital subtraction angiography (DSA) has also been shown, with studies reporting sensitivity rates comparable to DSA [2, 3]. MRI-based neurovascular vessel imaging has two goals: the anatomico-morphological depiction and vessel wall imaging including delineation of contrast enhancement within the vessel wall. There is a broad spectrum of potential clinical indications in neuroradiology with the most common being unruptured intracranial aneurysms, intracranial stenosis, and the broad spectrum of cerebral vasculitis, particularly large- to medium-size-vessel vasculitis. The goals of this study were i) to establish a neurovascular vessel imaging protocol at 7T MRI and ii) integrate this imaging protocol into clinical routine. Here, we report the results of sequence implementation and optimization as well as first clinical examples.Methods
First, sequences were optimized in healthy volunteers to establish a dedicated 7T MRI neurovascular imaging protocol. Image quality was assessed for each sequence by two experienced imaging experts according to a previously published protocol [4]. The sequence optimization was focused on T1 spin-echo (T1 SE) and T1-weighted “Sampling Perfection with Application optimized Contrasts using different flip angle Evolution” (T1 SPACE). In the T1 SPACE, we evaluated the effect of blood suppression, different TRs (440 ms, 700 ms, 1200 ms), TEs (4 ms, 13 ms), and turbo factors (TF) (20,40,60,69). Furthermore, we studied the impact of using an RF train with either variable or constant flip angles. Finally, the usage of a pulse that restores the longitudinal magnetization was tested. CAPIRINHA was used to shorten scan time with a 3x2(1) acceleration. In the T1 SE sequence, we evaluated different TR (440 ms, 700 ms, 1100 ms), blood suppression (on/off), flip angle combinations (90°/135°, 71°/135°), and number of averages (1,2). Once an optimal protocol was reached, we examined 28 patients at 7T (MAGNETOM Terra, Siemens Healthcare, Erlangen, Germany) using a single channel transmit and 32-channel receive head coil (Nova Medical, Wilmington, MA, USA). Subsequently, all patients received standard contrast-enhanced imaging at 3T (MAGNETOM Prisma, Siemens Healthcare, Erlangen, Germany) equipped with a 64-channel head coil of the vendor. Selected patients were scanned again at 7T approximately 15 minutes after the 3T MRI to assess persisting contrast enhancement. Written informed consent had been obtained from all patients prior to examination.Results
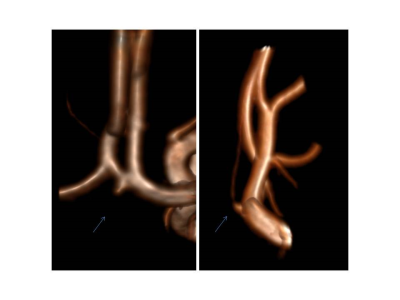
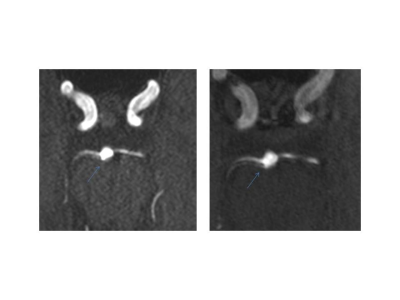
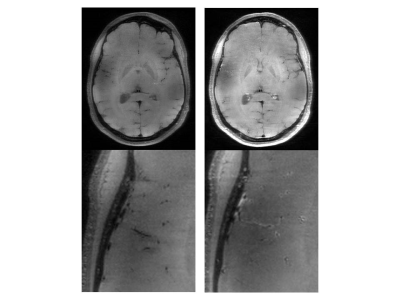
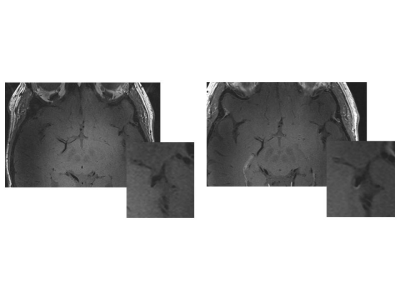
In the T1 SPACE, the combination TR/TE = 1200/13 ms provided the best contrast between the vessel wall structures and dark blood. Variable flip angle pulses resulted in the best image quality, while the longitudinal magnetization restoration pulse improved homogeneity in the T1-weighted contrast. A TF of 40 provided good image quality while maintaining the total acquisition time below 10 minutes. In the T1 SE, a TR of 700 ms and flip angles 71°/135° provided optimal contrast and T1-weighting which aided in clearly delineating the vessel wall structures. Blood suppression improved the T1 SE protocol as it suppresses in-flowing, unsaturated spins. Two averages improved the SNR and resulted in a total acquisition time of around 7 minutes for the final protocol. The final clinical routine protocol consisted of a set of sequences for both anatomico-morphological and vessel wall imaging: time-of-flight angiography (ToF), 3D MP-RAGE, susceptibility-weighted imaging (SWI), T2 turbo spin-echo (T2 TSE) sequences, T1 SE, and T1 SPACE sequences. The post-contrast measurements focused on T1 SE and T1 SPACE sequences. All sequence parameters are summarized in Table 1. We examined 28 patients with suspected or confirmed neurovascular pathologies (confirmed or suspected unruptured intracranial aneurysm, n=24; arteriovenous malformation, n=2; suspected vasculitis n=2). All 28 examinations were scanned without interruption or side effects. Anatomico-morphological sequences provided excellent depiction of the arterial vessels structures and suspected pathological findings suggesting superiority to 3T imaging in differentiation of true pathologies from normal vessel variants (Figures 1 and 2). Sequences for vessel wall imaging provided reproducible delineation of vessel walls for the whole brain (T1 SPACE) and selected regions of interest (T1 SE). Contrast enhancement was detectable at 7T MRI approximately 15 minutes after the completion of contrast-enhanced 3T imaging (Figure 3 and 4).Discussion and Conclusion
The presented optimization steps resulted in a clinically applicable neurovascular vessel imaging protocol at 7T. It appears that 7T MRI presents a powerful tool for of anatomico-morphological characterization and vessel wall delineation. Especially a subgroup of patients with ambiguous findings at 1.5T and 3T MRI may benefit from ultra-high-field MR imaging at 7T. These findings will be validated in a larger cohort in a future study. In summary, integration of ultra-high-field MR neurovascular imaging at 7T into clinical routine is feasible and showed first results in anatomico-morphological depiction, and vessel wall enhancement.Acknowledgements
No acknowledgement found.References
1. Wrede, K.H., et al., Non-enhanced MR imaging of cerebral aneurysms: 7 Tesla versus 1.5 Tesla. PLoS One, 2014. 9(1): p. e84562.
2. Monninghoff, C., et al., Evaluation of intracranial aneurysms with 7 T versus 1.5 T time-of-flight MR angiography - initial experience. Rofo, 2009. 181(1): p. 16-23.
3. Wrede, K.H., et al., Non-enhanced magnetic resonance imaging of unruptured intracranial aneurysms at 7 Tesla: Comparison with digital subtraction angiography. Eur Radiol, 2017. 27(1): p. 354-364.
4. Springer, E., et al., Comparison of Routine Brain Imaging at 3 T and 7 T. Invest Radiol, 2016. 51(8): p. 469-82.
Figures