4147
Generating virtual brains for MRI-based 3D cerebral blood flow simulations1Department of Engineering Science, University of Oxford, Oxford, United Kingdom, 2Institute of Radiopharmaceutical Cancer Research, Helmholtz-Zentrum Dresden-Rossendorf, Dresden, Germany, 3Department of Radiology and Nuclear Medicine, Amsterdam University Medical Center, Amsterdam, Netherlands
Synopsis
Human brain perfusion simulations have been limited to less than five patient-specific cases. We propose a pipeline based on MRI to overcome this limitation. Computational geometry is adjusted using T1-weighted MRI, and the perfusion model parameters are tuned based on arterial spin labeling perfusion MRI. A cohort of 75 patients is used to demonstrate that the pipeline is suitable to generate virtual patients with statistically accurate and precise cerebral blood flow maps. Our findings encourage future studies on in silico clinical trials using similar virtual cohorts to improve ischaemic stroke interventions.
Introduction
The IN Silico clinical trials for acute Ischaemic STroke (INSIST) consortium1 suggests that in silico trials could accelerate medical device and drug development cost-efficiently and aims to develop a software suite to simulate stroke interventions (thrombolysis and thrombectomy). The proposed simulations may help to focus stroke-intervention trials by optimising drugs and devices in virtual patients. Brain tissue perfusion computation plays a key role in stroke simulation, as well as in the risk and benefit estimation associated with the intervention.Several computational studies have presented porous circulation models to estimate Cerebral Blood Flow (CBF) corresponding to healthy2 and occluded arteries3. Configuring these models requires knowledge of typical (patho-)physiological parameters. Such parameter sets, including reconstructed brain geometries (volumetric meshes) and grey & white matter (GM & WM) permeabilities4,5, characterise virtual patients but are challenging to obtain. For this reason, computational perfusion studies were previously often limited to a single virtual patient2,3, which is insufficient to simulate complex scenarios encountered in intervention trials incorporating thousands of patients. The present study sets out to overcome this issue, by combining T1-weighted and ASL perfusion MRI with optimisation and simulations to generate virtual patients for CBF predictions in healthy and occluded scenarios.
Methods
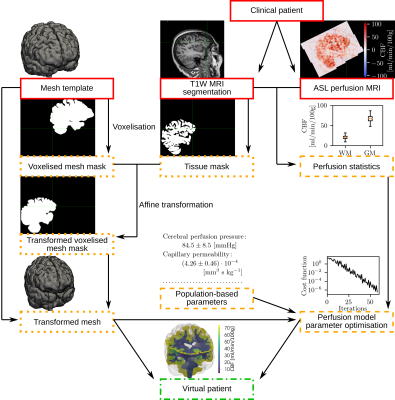
Figure 1 shows a schematic overview of the virtual patient generation pipeline. Data for 75 patients (age 68±8 years, 56% female) are drawn from the European Prevention of Alzheimer’s Dementia (EPAD)6 v500.0 release, from a single 3T Achieva scanner (Philips Healthcare). T1-weighted and ASL perfusion images in the native space are processed with ExploreASL7 to obtain GM and WM masks (partial volume larger than 50%) and CBF maps, respectively. A tetrahedral mesh capturing GM & WM is affine registered with FSL FLIRT8 to the T1w image based on tissue masks obtained by voxelising a mesh template3. These steps correspond to the dotted boxes in Figure 1. CBF statistics — including mean values, and 20th and 80th percentile values — are obtained in GM & WM using partial volume correction. Because the simulations predict time-averaged CBF distributions; and the ASL MRI CBF maps can contain artificial vascular peaks and valleys, only the 20th and 80th percentile values are considered as robust estimates of the minimum and maximum CBF values. Parameters of the perfusion simulator detailed in the following paragraphs are determined to match patient-specific CBF values. These steps correspond to the dashed boxes in Figure 1.The computational perfusion model describes CBF in the microcirculation based on a set of partial differential equations: $$ \nabla \cdot (K_i \nabla p_i) - \sum_{j=1}^3 \beta_{ij} (p_i-p_j) = 0. $$ Here, $$$i=1,~2,~3$$$ represents the arteriole, capillary, and venule compartments (the Einstein summation notation is not used). E.g., $$$p_1$$$ and $$$K_1$$$ are the arteriole pressure and permeability tensor, and $$$\beta_{12}$$$ is the arteriole-capillary coupling coefficient. Each permeability tensor $$$K_i$$$ is characterised by a single scalar $$$k_i$$$ as described previously6. In the healthy case, constant arteriole $$$(p_{1,bc})$$$ and venous pressures $$$(p_{3,bc})$$$ are prescribed as boundary conditions on the cortical surface, such that the cerebral perfusion pressure is $$$CPP=(p_{1,bc}-p_{3,bc})$$$.
Several model parameters are randomised to simulate physiological variability whereas others are optimised to match real patients' CBF statistics6. The randomised model parameters are (mean±SD):
- $$$CPP=84.0\pm8.5$$$ [mmHg] (set based on9);
- $$$k_2=(4.26\pm0.46)\cdot 10^{-4}$$$ [mm3 s kg-1] (set based on4);
- $$$k_3 / k_1=2.0\pm0.2$$$ (set based on2,3);
- $$$\beta_{12}^{GM}=(1.17\pm0.27)\cdot 10^{-6}$$$ [Pa-1 s-1] (set based on2,3,4,5), and
- $$$\beta_{23}^{GM}=(4.22\pm1.02)\cdot 10^{-6}$$$ [Pa-1 s-1] (set based on2,3,4,5).
- $$$k_1=0.98\pm0.25\cdot 10^{-4}$$$ [mm3 s kg-1], and
- $$$\beta_{ij}^{GM} / \beta_{ij}^{WM}=3.68\pm0.91$$$.
Results and Discussion
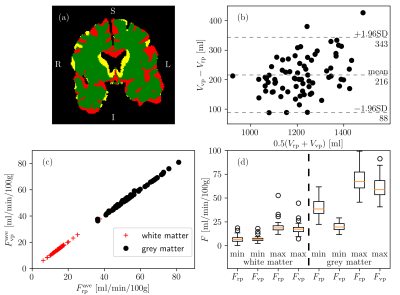
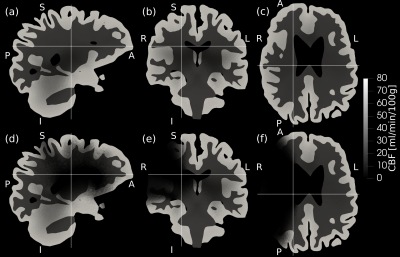
75 virtual brains were generated so that every virtual brain had a different geometry and CBF distribution that is based on the corresponding real patient. The overlap between the patient’s tissue mask and the transformed voxelised mesh template was consistently higher than 95% but the spatial resolution of the gyri and sulci of the mesh template was too low (Figure 2a). As a result, the tissue volume was overestimated by the transformed mesh on average by 20% as indicated by the Bland-Altman plot comparing the brain volume of virtual and real patients (Figure 2b). Figures 2c-d show that the CBF statistics of virtual and real brains were similar, suggesting that the optimisation was successful. Example computed CBF maps corresponding to a virtual patient are shown in Figure 3. Examples of healthy and occluded scenarios are visualised in Figure 3a-c and Figure 3d-f, respectively.Conclusion
Our promising findings show good CBF agreement between the virtual and real brains. Although the transformed meshes appear suitable to incorporate the effects of volumetric changes, they cannot account for morphological variability because of the under-resolved template and the affine-only registration. Future pipelines may be improved with nonlinear registration and a more accurate mesh template. These findings are a promising contribution to comprehensive in silico trials of ischemic stroke interventions.Acknowledgements
This work was supported by the European Union‘s Horizon 2020 research and innovation programme, the INSIST project, under grant agreement No 777072. TIJ and SJP are grateful to the members of the INSIST consortium for useful discussions and their sustained support. The EPAD data were obtained with support from the following EU/EFPIA Innovative Medicines Initiatives (1 and 2) Joint Undertakings: EPAD grant no. 115736, AMYPAD grant no. 115952. HJMMM is supported by the Dutch Heart Foundation (2020T049), and by the Eurostars-2 joint programme with co-funding from the European Union Horizon 2020 research and innovation programme, provided by the Netherlands Enterprise Agency (RvO).
References
- P. R. Konduri, H. A. Marquering, E. van Bavel, C. B. L. Majoie, and the INSIST Investigators. In-silico trials for treatment of acute ischemic stroke. Frontiers in Neurology, 11:558125, 2020.
- E. Hodneland, E. Hanson, O. Sævareid, G. Nævdal, A. Lundervold, V. Šoltészová, A. Z. Munthe-Kaas,A. Deistung, J. R. Reichenbach, and J. M. Nordbotten. A new framework for assessing subject-specific whole brain circulation and perfusion using MRI-based measurements and a multi-scale continuous flow model. PLoS Computational Biology, 15(6):e1007073, 2019.
- T. I. Józsa, R. M. Padmos, N. Samuels, W. K. El-Bouri, A. Hoekstra, S. J. Payne. A porous circulation model of the human brain for in silico clinical trials in ischaemic stroke. Interface Focus, 11:20190127, 2020.
- W. K. El-Bouri, S. J. Payne. Multi-scale homogenization of blood flow in 3-dimensional human cerebral microvascular networks. Journal of Theoretical Biology, 380, 40-47, 2015.
- W. K. El-Bouri, A. MacGowan, T. I. Józsa, M. J. Gounis, S. J. Payne. Modelling the impact of clot fragmentation on the microcirculation after thrombectomy. bioR$$$\chi$$$iv preprint, 2020.
- C. W. Ritchie, J. L. Molinuevo, L. Truyen, A. Satlin, S. Van der Geyten, S. Lovestone and the EPAD Investigators. Development of interventions for the secondary prevention of Alzheimer's dementia: the European Prevention of Alzheimer's Dementia (EPAD) project. The Lancet Psychiatry, 3(2), 2016.
- H. J. M. M. Mutsaerts, J. Petr, P. Groot, P. Vandemaele, S. Ingala, A. D. Robertson, L. Václavů, I. Groote, H. Kuijf, F. Zelaya, O. O’Daly, S. Hilal, A. M. Wink, I. Kant, M. W. A. Caan and others. ExploreASL: An image processing pipeline for multi-center ASL perfusion MRI studies, NeuroImage, 219:117031, 2020.
- M. Jenkinson, P. Bannister, J. M. Brady and S. M. Smith. Improved optimisation for the robust and accurate linear registration and motion correction of brain images. NeuroImage, 17(2), 825-841, 2002.
- K. Malhotra, N. Ahmed, A. Filippatou, A. H. Katsanos, N. Goyal, K. Tsioufis, E. Manios, M. Pikilidou, P. D. Schellinger, A. W. Alexandrov, A. V. Alexandrov, G. Tsivgoulis. Association of Elevated Blood Pressure Levels with Outcomes in Acute Ischemic Stroke Patients Treated with Intravenous Thrombolysis: A Systematic Review and Meta-Analysis. Journal of Stroke, 21(1):78-90, 2019.
Figures