4146
Quantification of hemodynamics of cerebral arteriovenous malformations after stereotactic radiosurgery using 4D Flow MRI1Department of Radiology, University of California San Diego, San Diego, CA, United States, 2Department of Radiation Medicine and Applied Sciences, University of California San Diego, San Diego, CA, United States, 3Department of Radiology, Stanford University, Stanford, CA, United States
Synopsis
This study evaluated the use of 4D flow MRI to assess hemodynamic changes to cerebral arteriovenous malformations after stereotactic radiosurgery (SRS). As a comparison, structural changes of AVMs were measured on T2W single shot fast spin echo (SSFSE) and time-of-flight (TOF) angiography. Hemodynamic changes, including reduction in feeding arterial flow and draining venous flow, preceded structural changes including arterial circumference and nidus volume for large arteriovenous malformations. This study demonstrated the potential utility of 4D flow MRI as a robust tool to supplement existing imaging techniques and measure early treatment response after stereotactic radiosurgery (SRS).
Introduction
In cerebral arteriovenous malformations (AVMs), the normal capillary bed is disrupted by aberrant angiogenesis (1). For small cerebral AVMs not amenable to surgery, stereotactic radiosurgery (SRS) is a standard treatment (2). SRS induces endothelial-intimal changes, thereby allowing the nidus to decrease in size and eventually obliterate (3). Traditionally, structural T2-weighted MRI has been used to monitor treatment response after SRS followed by digital subtraction angiography (DSA) to confirm obliteration (4, 5). However, these structural changes may take many years to occur (6). In the interim, hemodynamic changes which may predispose AVMs to rupture remain unknown (7). 4D flow MRI is an emerging phase-contrast technology which has demonstrated reproducibility (8) and good correlation with pre-existing techniques for measuring cerebral blood flow in healthy patients (9, 10). This study aimed to assess the use of 4D flow MRI to measure early treatment response of cerebral AVMs after SRS.Methods
This was a retrospective, observational study with serial measurements performed pre-SRS and post-SRS at multiple time points from 1 to 27 months. A total of 14 patients, 6 females and 8 males were included in this study. As part of a standard protocol, patients underwent 4D flow MRI with a velocity-encoding of 150-200 cm/s, 3D Time-of-flight (TOF) MRA, and T2-weighted single shot fast spin echo (SSFSE) MRI on a 3T GE Discovery 750 MRI scanner (GE Healthcare, Milwaukee, Wisconsin). AVM nidus volume was measured on T2-weighted MRI. AVMs which were larger than 3 cm in length, height, width, and volume ≥ 27 cm3 were classified as large. Posthoc 2D cross-sectional flow and circumference were measured for the dominant feeding artery, dominant draining vein and corresponding contralateral artery. Pulsatility index was calculated for each arterial measurement as (peak systolic flow – minimum diastolic flow)/(peak systolic flow).Wilcoxon signed-rank tests were used to compare differences in flow, circumference, and pulsatility between the feeding artery and the contralateral artery both before and after SRS; and differences in nidus size and flow and circumference of the feeding artery and draining vein before and after SRS. A secondary analysis assessed differences in flow and volume between small (volume < 27 cm3) and large (volume >= 27 cm3) AVMs using a Wilcoxon signed-rank test.
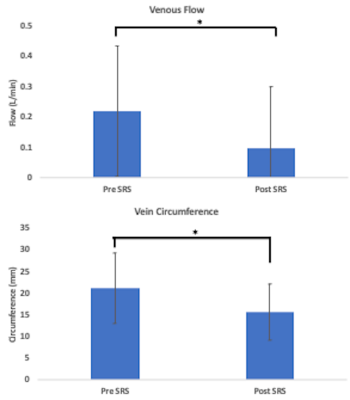
Results
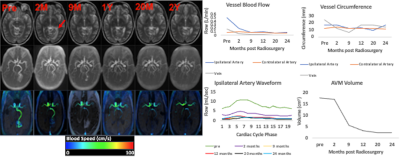
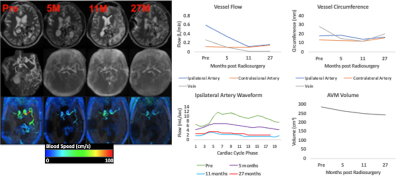
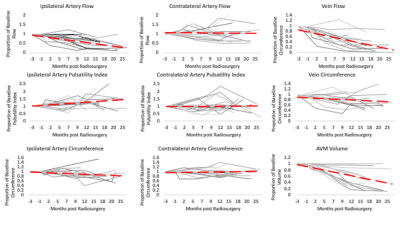
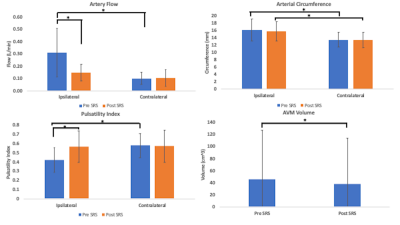
Figures 1 and 2 illustrate structural and hemodynamic changes for two patients, one with a small volume AVM and one with a large volume AVM, respectively. Figure 3 illustrates aggregate hemodynamic and structural changes over time for all patients in this study. Arterial flow (L/min) decreased in the primary feeding artery (0.1 ± 0.07 vs 0.3 ± 0.2; p < 0.05) and normalized in comparison to the contralateral artery (mean: 0.1 ± 0.07 vs 0.1 ± 0.05; p = 0.068). Arterial circumference did not decrease significantly in the primary feeding artery (15.7 ± 2.7 vs 16.1 ±3.1; p = 0.358) and remained significantly higher than in the contralateral artery (15.7 ± 2.7 vs 13.4±2.2; p <0.05) despite the changes in flow. Flow decreased in the draining vein (mean: 0.1 ± 0.2 vs 0.2 ± 0.2; p < 0.05), and the circumference of the draining vein also decreased (mean: 16.1 ± 8.3 vs 15.7 ± 6.7; p < 0.05). AVM volume decreased after SRS (mean: 45.3 ± 84.8 vs 38.1 ± 78.7; p < 0.05). (Figure 4, 5) However, in a subset analysis of small and large AVMs, volume (cm^3) did not decrease significantly for large AVMs (pre-SRS: 138.1 ± 121.8 vs. post-SRS: 125.4 ± 111.9) (p = 0.181), but there was a significant reduction in volume (cm^3) for small AVMs (pre-SRS: 6.7 ± 10.7 vs. post-SRS: 3.2 ± 5.9) (p < 0.05).Discussion
In this study, hemodynamic parameters including ipsilateral artery flow and venous flow changed significantly after SRS prior to structural parameters including arterial circumference as well as AVM nidus volume for large AVMs. These results extend prior findings from a case study in a single subject (11). As demonstrated in previous studies(12-14), this study showed pulsatility was significantly lower in the feeding artery of the AVM than the contralateral artery prior to treatment. Pulsatility significantly increased within the feeding artery post-SRS and normalized in comparison to the contralateral artery likely secondary to structural changes induced by SRS including hyaline sclerosis and endothelial-intimal damage (3).Conclusions
4D flow MRI demonstrates early hemodynamic changes in cerebral AVMs treated with SRS, including reduction in feeding arterial and draining venous blood flow. These hemodynamic changes may precede structural changes such as circumference of the feeding artery and AVM nidus volume for large AVMs. 4D flow MRI can potentially serve as a powerful adjunct to routine MRI/MRA in monitoring early treatment response of cerebral AVMs following SRS.Acknowledgements
This work was partially supported by the Radiological Society of North America (RSNA) Resident Research Grants RR1965 (A.S.); RR1879 (T.R.);RSNA Medical Student Research Scholarship RMS1825 (S.S.); MedGap (S.S); National Institutes of Health (NIH) grants T32 EB005970 (T.R.), #1KL2TR001444 (JAH-G), R01CA238783-01 (JAH-G). The content is solely the responsibility of the authors and does not necessarily represent the official views of the RSNA or NIH. Dr. Hsiao is founder and consultant of Arterys whose technology was used in this study to capture 4D Flow MRI images. All other authors including myself have no conflicts of interest.References
1. Jabbour MN, Elder Jb Fau - Samuelson CG, Samuelson Cg Fau - Khashabi S, Khashabi S Fau - Hofman FM, Hofman Fm Fau - Giannotta SL, Giannotta Sl Fau - Liu CY, et al. Aberrant angiogenic characteristics of human brain arteriovenous malformation endothelial cells. (1524-4040 (Electronic)).
2. Gilbo P, Zhang I, Knisely J. Stereotactic radiosurgery of the brain: a review of common indications. Chinese Clinical Oncology; Vol 6, Supplement 2 (September 2017): Chinese Clinical Oncology (Advances in Stereotactic Radio Surgery-Guest Editors: Kevin L M Chua, David B H Tan, Melvin L K Chua, Simon S Lo). 2017.
3. Schneider BF, Eberhard Da Fau - Steiner LE, Steiner LE. Histopathology of arteriovenous malformations after gamma knife radiosurgery. (0022-3085 (Print)).
4. Chen JC, Mariscal L, Girvigian MR, Vanefsky MA, Glousman BN, Miller MJ, et al. Hypofractionated stereotactic radiosurgery for treatment of cerebral arteriovenous malformations: outcome analysis with use of the modified arteriovenous malformation scoring system. (1532-2653 (Electronic)).
5. Starke RM, Kano H, Ding D, Lee JY, Mathieu D, Whitesell J, et al. Stereotactic radiosurgery for cerebral arteriovenous malformations: evaluation of long-term outcomes in a multicenter cohort. (1933-0693 (Electronic)).
6. Kano H, Kondziolka D Fau - Flickinger JC, Flickinger Jc Fau - Yang H-c, Yang Hc Fau - Flannery TJ, Flannery Tj Fau - Awan NR, Awan Nr Fau - Niranjan A, et al. Stereotactic radiosurgery for arteriovenous malformations, Part 3: outcome predictors and risks after repeat radiosurgery. (1933-0693 (Electronic)).
7. Shaligram SS, Winkler E, Cooke D, Su H. Risk factors for hemorrhage of brain arteriovenous malformation. CNS Neuroscience & Therapeutics. 2019;25(10):1085-95. doi: 10.1111/cns.13200.
8. Wen B, Tian S, Cheng J, Li Y, Zhang H, Xue K, et al. Test-retest multisite reproducibility of neurovascular 4D flow MRI. (1522-2586 (Electronic)).
9. Cebral JR, Putman Cm Fau - Alley MT, Alley Mt Fau - Hope T, Hope T Fau - Bammer R, Bammer R Fau - Calamante F, Calamante F. Hemodynamics in Normal Cerebral Arteries: Qualitative Comparison of 4D Phase-Contrast Magnetic Resonance and Image-Based Computational Fluid Dynamics. (0022-0833 (Print)).
10. Meckel S, Leitner L, Bonati LH, Santini F, Schubert T, Stalder AF, et al. Intracranial artery velocity measurement using 4D PC MRI at 3 T: comparison with transcranial ultrasound techniques and 2D PC MRI. Neuroradiology. 2013;55(4):389-98. doi: 10.1007/s00234-012-1103-z.
11. Li CQ, Hsiao A, Hattangadi-Gluth J, Handwerker J, Farid N. Early Hemodynamic Response Assessment of Stereotactic Radiosurgery for a Cerebral Arteriovenous Malformation Using 4D Flow MRI. American Journal of Neuroradiology. 2018.
12. Stuer C, Ikeda T Fau - Stoffel M, Stoffel M Fau - Luippold G, Luippold G Fau - Sakowitz O, Sakowitz O Fau - Schaller K, Schaller K Fau - Meyer B, et al. Norepinephrine and cerebral blood flow regulation in patients with arteriovenous malformations. (1524-4040 (Electronic)).
13. Diehl RR, Linden D Fau - Lucke D, Lucke D Fau - Berlit P, Berlit P. Phase relationship between cerebral blood flow velocity and blood pressure. A clinical test of autoregulation. (0039-2499 (Print)).
14. Schnell S, Wu C, Ansari SA. 4D MRI flow examinations in cerebral and extracerebral vessels. Ready for clinical routine? Current opinion in neurology. 2016;29(4):419-28. doi: 10.1097/WCO.0000000000000341. PubMed PMID: PMC4939804.
Figures