4141
Using ASL perfusion images for spatial normalization in a pediatric population with craniosynostosis.1Erasmus University Medical Center, Rotterdam, Netherlands, 2Amsterdam UMC, Amsterdam, Netherlands, 3Institute of Radiopharmaceutical Cancer Research, Dresden, Germany
Synopsis
Spatial normalization is an important step for image processing and quantification of regional brain perfusion values using arterial spin labeling (ASL) MRI and is typically performed via high-resolution structural scans. Structural segmentation and/or registration is complicated when gray-white matter T1w contrast is low and changing in early phases of myelination in newborns. Craniosynostosis is a condition where the decision for surgical treatment in the first years of life is supported by brain imaging. In this study, we investigate if ASL CBF image contrast can be directly used for spatial normalization, in both healthy controls and a non-syndromic type of craniosynostosis.
Spatial normalization is an important step for image processing and quantification of regional brain perfusion values using arterial spin labeling (ASL) MRI and is typically performed via high-resolution structural scans. Structural segmentation and/or registration is complicated when gray-white matter T1w contrast is low and changing in early phases of myelination in newborns. This is complicated even further in children with craniosynostosis, whose skulls are deformed in various nonlinear ways. 1-3 Both situations are present in scans of children with craniosynostosis. Craniosynostosis is a condition where skull sutures close prematurely and the decision for surgical treatment in the first years of life is supported by brain imaging.4-7 In this study, we investigate if the ASL CBF image contrast can be directly used for spatial normalization, in both healthy controls and trigonocephaly patients - a non-syndromic type of craniosynostosis.
METHODS
Between 2018 and 2020, 36 trigonocephaly patients (median age 6 months, IQR 4, 11 females) and 16 healthy controls (median age 10 months, IQR 66, 10 females) underwent brain MRI with ASL to measure cerebral perfusion. Image processing and evaluation was performed with ExploreASL.8-10 After aligning, motion correcting, and quantifying the ASL images [ref xASL], we compared four registration approaches: 1) Registration via segmentation and spatial normalization of the T1w images (regT1w); and a direct registration of ASL to MNI using 2) rigid-body (regASLrigid), 3) affine (regASLaffine), or 4) non-linear Direct Cosine Transform (DCT) transformation (regASLdct). For regT1, the T1w image was segmented and registered using SPM12 with a 1-year-old infants template (NITRC, version V2.3).11 The M0 and T1w images were rigidly aligned and a joint transformation from ASL native space to MNI common space was applied. For the regASLrigid, regASLaffine, regASLdct, a pseudo-CBF image was constructed in the MNI space by assigning GM and WM CBF voxels of 60 and 20 mL/min/100 g, respectively. The native ASL CBF image was then registered with the pseudo-CBF image using rigid-body (2), affine (3), and DCT transformations (4), respectively. Registration performance was expressed by the Tanimoto coefficient (TC) and compared quantitatively by a linear mixed model.
RESULTS
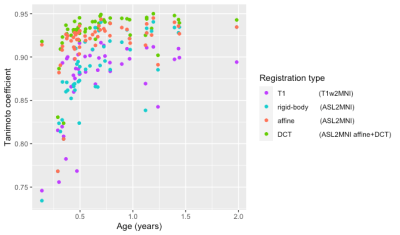
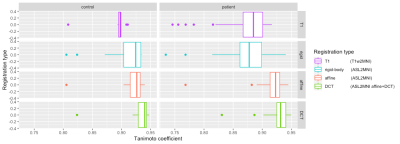
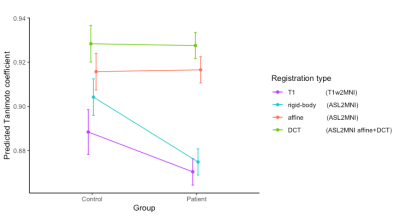
For the total cohort, regASLdct showed the highest TC score (Figure 1). Registration performance did not differ between registration types in patients and controls (Figure 2). For both patients and controls, regASLdct showed the highest registration performance. RegASLaffine outperformed regT1 (TC improvement of 0.03, p-value < 0.05) and regASLdct outperformed regT1 (TC improvement of 0.04 p-value < 0.05). The interaction plot (Figure 3) shows that regASLdct and regASLaffine have a higher TC in comparison to regASLrigid of regT1in both patients and controls. TC will decrease using the registration method regASLrigid and regT1 in patients.
DISCUSSION
We have shown that direct registration to MNI space using ASL CBF as image contrast outperforms spatial normalization based on T1w segmentation in both patients and controls. The non-linear registration outperformed both rigid and affine registration. While better results were shown for the control group, the difference between regT1 and regASL was even higher in patients showing the increasing importance of this method in a cohort with poor GM-WM T1w contrast and large skull deformations. One main limitation is that we tested whole-brain alignment only. A more thorough validation is needed to demonstrate if this registration method allows regional CBF evaluation in MNI space using gray and white matter masks. Many ASL studies in neonates, those two included, rely on manual ROI delineation.12, 13 Using the proposed method to automate ASL analysis gives tools to delineate the neuroanatomical information leading to a higher reproducibility of results. For trigonocephaly patients it is therefore beneficial to use regASLdct.
CONCLUSION
In conclusion, the ASL CBF contrast is a viable spatial normalization alternative if structural images are not available or have poor contrast. The results of this study may be an important step for the feasibility of future large pediatric ASL studies.
Acknowledgements
No acknowledgement found.References
1. Knickmeyer RC, Gouttard S, Kang C, et al. A structural MRI study of human brain development from birth to 2 years. J Neurosci 2008;28:12176-12182
2. Holland D, Chang L, Ernst TM, et al. Structural growth trajectories and rates of change in the first 3 months of infant brain development. JAMA Neurol 2014;71:1266-1274
3. Dubois J, Dehaene-Lambertz G, Kulikova S, et al. The early development of brain white matter: a review of imaging studies in fetuses, newborns and infants. Neuroscience 2014;276:48-71
4. de Jong T, Bannink N, Bredero-Boelhouwer HH, et al. Long-term functional outcome in 167 patients with syndromic craniosynostosis; defining a syndrome-specific risk profile. J Plast Reconstr Aesthet Surg 2010;63:1635-1641
5. Spruijt B, Joosten KF, Driessen C, et al. Algorithm for the Management of Intracranial Hypertension in Children with Syndromic Craniosynostosis. Plast Reconstr Surg 2015;136:331-340
6. Maliepaard M, Mathijssen IM, Oosterlaan J, et al. Intellectual, behavioral, and emotional functioning in children with syndromic craniosynostosis. Pediatrics 2014;133:e1608-1615
7. Johnson D, Wilkie AO. Craniosynostosis. Eur J Hum Genet 2011;19:369-376
8. Mutsaerts HJ, van Osch MJ, Zelaya FO, et al. Multi-vendor reliability of arterial spin labeling perfusion MRI using a near-identical sequence: implications for multi-center studies. Neuroimage 2015;113:143-152
9. Mutsaerts HJ, Steketee RM, Heijtel DF, et al. Inter-vendor reproducibility of pseudo-continuous arterial spin labeling at 3 Tesla. PLoS One 2014;9:e104108
10. Mutsaerts H, Petr J, Groot P, et al. ExploreASL: An image processing pipeline for multi-center ASL perfusion MRI studies. Neuroimage 2020;219:117031
11. Shi F, Yap PT, Wu G, et al. Infant brain atlases from neonates to 1- and 2-year-olds. PLoS One 2011;6:e18746
12. De Vis JB, Petersen ET, de Vries LS, et al. Regional changes in brain perfusion during brain maturation measured non-invasively with Arterial Spin Labeling MRI in neonates. Eur J Radiol 2013;82:538-543
13. Miranda MJ, Olofsson K, Sidaros K. Noninvasive measurements of regional cerebral perfusion in preterm and term neonates by magnetic resonance arterial spin labeling. Pediatr Res 2006;60:359-363
Figures