4140
Effect of Small Vessel Disease Burden on Collateral Perfusion in Symptomatic Large Vessel Stenosis or Occlusion1the First Medical Center, Chinese PLA General Hospital, Beijing, China
Synopsis
Total SVD burden were not correlated with collateral perfusion, but perivascular space could impact collaterals.
INTRODUCTION
The effect of small vessel disease (SVD) burden on collateral circulation in acute ischemic stroke reached different conclusions. We sought to identify whether the SVD burden could also impact collateral circulation in symptomatic ICA/MCA severe stenosis or occlusion patients.METHODS
All included patients underwent pseudo-continuous arterial spin labeling (pCASL) with post-labeling delay (PLD) of 1.5s and 2.5s. The total SVD burden were assessed by four separate features: white matter hyperintensity, perivascular space (PVS), lacunes and cerebral microbleed. Cerebral blood flow (CBF) of MCA territory was calculated by manually drawing region of interest. Collateral perfusion was defined as (CBF 2.5s minus CBF 1.5s) at lesion side minus (CBF 2.5s minus CBF 1.5s) at normal side1.RESULTS
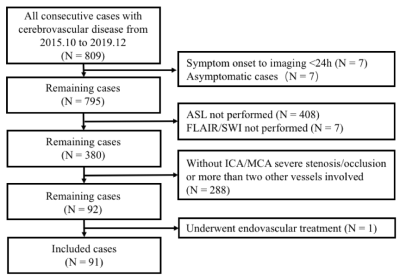
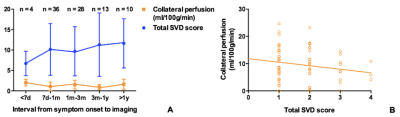
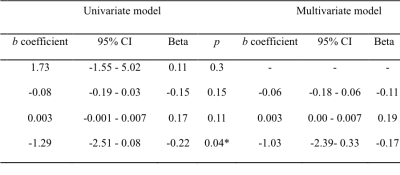
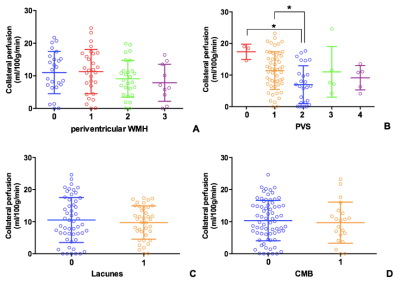
A total of 91 patients with symptomatic ICA/MCA severe stenosis/occlusion met the inclusion criteria (Figure 1). Compared with patients with no SVD burden (N = 24), those with any SVD (N = 67) were older (t = -2.56, p = 0.01), and showed higher incidence of diabetes mellitus (χ2 = 10.71, p = 0.001). Furthermore, the mean CBF at PLD 2.5s in patients with any SVD was lower than those without SVD (t = 3.01, p = 0.003). Collateral perfusion was significantly correlated with total SVD score in univariate linear regression (b = -1.29, p = 0.04) (Figure 2). However, such correlation disappeared after adjusted for age and interval from symptom onset to imaging (b = -1.03, p = 0.14) (Figure 3). In the subgroup analysis, PVS could still significantly impact collateral circulation (F = 3.21, p = 0.02) after adjusted for age, sex and interval from symptom onset to imaging (Figure 4).DISCUSSION
We found that patients with any SVD were older than those without SVD, which was similar to previous findings2. Moreover, we found that the incidence of diabetes mellitus was higher in patients with SVD. It has been acknowledged that diabetes mellitus could affect both large and small vessels. Funnell et al. suggested that diabetes mellitus are an important risk factor for SVD, which was consistent with our findings3. Recently, a study by Eker et al. found no correlation between SVD burden and collateral circulation in patients with acute stroke4. However, the study by Lin et al. found opposite results5. In our study, the collateral perfusion were higher in patients without SVD burden. In univariate linear regression analysis, SVD burden could impact the collateral recruitment. However, after adjusted for age and interval from symptom onset to imaging, such association disappeared. Our results indicated that after a relatively long interval from symptom onset to imaging, the collateral perfusion may be stabilized to an appropriate level to compensate for cerebral perfusion, which could not be impacted by SVD burden. By using general linear model, after adjusted for age, sex and interval from symptom onset to imaging, the PVS could still affect the collateral perfusion, which indicated that PVS may serve as one long-term factor.CONCLUSION
Our results suggested that collateral circulation may not be affected by total SVD burden in chronic symptomatic large vessel stenosis or occlusion. However, PVS may affect the collateral perfusion after a relatively long time from symptom onset. Our results suggested that PVS could be one of the possible therapeutic targets for improvement of collateral circulation.Acknowledgements
No acknowledgement found.References
1. JH Lyu, N Ma, DS Liebeskind et al. Arterial spin labeling MRI estimation of antegrade and collateral flow in unilateral middle cerebral artery stenosis. Stroke. 2016; 47(2): 428–433.
2. S Koton, ALC. Schneider, BG Windham et al. Microvascular brain disease progression and risk of stroke: the ARIC study. Stroke. 2020;51(11):3264-3270.
3. C Funnell, MM Doyle-Waters, S Yip et al. What is the relationship between type 2 diabetes mellitus status and the neuroradiological correlates of cerebral small vessel disease in adults? Protocol for a systematic review. Syst Rev. 2017;6(1):7.
4. OF Eker, L Rascle, TH Cho et al. Does small vessel Disease burden impact collateral circulation in ischemic stroke treated by mechanical thrombectomy? Stroke. 2019;50:1582-1585.
5. MP Lin, TG Brott, DS Liebeskind et al. Collateral recruitment is impaired by cerebral small vessel disease. Stroke. 2020;51:027661-00.
Figures