4134
Neuropathologic correlates of cerebral microbleeds in community-based older adults1Department of Computer Science, Illinois Institute of Technology, Chicago, IL, United States, 2Rush Alzheimer's Disease Center, Rush University Medical Center, Chicago, IL, United States, 3Department of Biomedical Engineering, Illinois Institute of Technology, Chicago, IL, United States
Synopsis
Cerebral microbleeds (CMBs) are common in older adults and have been linked to hypertension, increased risk of stroke, and cognitive decline. There is also evidence of an association between CMBs and cerebral amyloid angiopathy (CAA) in studies involving clinical populations and patients with intracerebral hemorrhage. However, it remains unclear what the neuropathologic correlates of CMBs are in community-based older adults. The aim of this study was to determine the neuropathologic correlates of CMBs in community-based older adults, and also to investigate the relationship between neuropathologies and the location of CMBs in the brain.
Introduction
Cerebral microbleeds (CMBs) are common in older adults and have been linked to hypertension1,2, increased risk of stroke3,4, and cognitive decline5-7. There is also evidence of an association between CMBs and cerebral amyloid angiopathy (CAA) in studies involving clinical populations8 and patients with intracerebral hemorrhage9,10. However, it remains unclear what the neuropathologic correlates of CMBs are in community-based older adults. The aim of this study was to determine the neuropathologic correlates of CMBs in community-based older adults, and also to investigate the relationship between neuropathologies and the location of CMBs in the brain.Methods
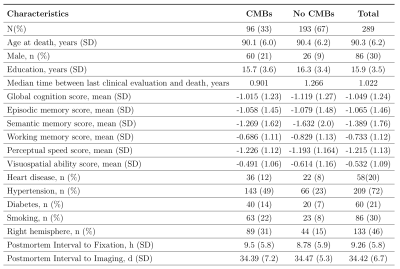
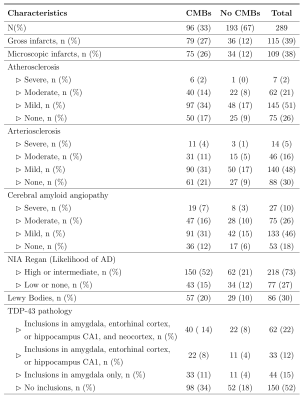
A total of 289 participants from two longitudinal cohort studies of aging, the Rush Memory and Aging Project11 and Religious Orders Study12, were included in this work. Participants received annual clinical evaluation and cognitive assessment, ex-vivo MRI, and neuropathologic examination (Fig.1). One brain hemisphere from each participant was scanned ex-vivo on a 3T MRI scanner using a 3D multi-echo gradient echo sequence with a voxel size of 1x1x1 mm3. Following ex-vivo MRI, a board-certified neuropathologist blinded to clinical and imaging findings performed detailed neuropathologic examination of all hemispheres. The pathologies assessed included: gross and microscopic infarcts, atherosclerosis, arteriolosclerosis, cerebral amyloid angiopathy (CAA), Alzheimer’s pathology, Lewy bodies, and TDP-43 (Fig.2).Manual identification of CMBs on ex-vivo T2*-weighted gradient echo images was done by an experienced rater blinded to all clinical and pathological information. CMBs were identified as small, dark features in brain tissue. Notable CMB mimics on ex-vivo T2*-weighted gradient echo images included air bubbles appearing near the edges of tissue, and in some cases blood deposits that remained in vessels postmortem. T2* maps and Frangi filtered T2*-weighted images were used to assist in building CMB detection criteria and to reduce false positives. The total number of CMBs as well as the number of CMBs per lobe were recorded for each participant.
Poisson regression was used to investigate the associations of the total and regional number of CMBs with neuropathologies, controlling for demographics and postmortem interval. A two step process was used for variable selection. In the first step, the number of CMBs was regressed against each neuropathology and demographic variable (independent variables) in single factor models. Variables achieving marginal significance were included in a combined model in the second step. Significance was set at p<0.05 and Bonferroni correction for multiple comparisons was used. A significance threshold of p<0.0083 was used after applying Bonferroni correction.
Results
A total of 507 microbleeds were identified in the 289 participants. At least one microbleed was found in 193 (66.78%) participants. Only 14 (4.84%) of the participants had more than 5 microbleeds present. Out of these, 356 CMBs were found in the frontal or parietal lobes (70.22%) with 59 (11.64%) CMBs present in the basal ganglia.The total number of CMBs was associated with CAA (beta=0.26, p<0.0001) and arteriosclerosis (beta=0.15, p=0.0043). The number of CMBs in the frontal lobe was also associated with CAA (beta=0.46, p<0.0001) and arteriosclerosis (beta=0.38, p<0.0001). The number of CMBs in other lobes did not show significant associations with neuropathologies after Bonferroni correction.
Discussion
This study provided robust evidence on associations of the number of CMBs with neuropathologically confirmed CAA and arteriolosclerosis in community-based older adults. While a link between CMBs and CAA in clinical populations has been established13,14, the link in the general population has remained unclear and prior work in this area has suffered from small sample sizes8. Recent studies on community cohorts found associations between amyloid burden on PET scans and number of CMBs15,16. This buildup of amyloid on arterial walls in CAA is believed to lead to the formation of microbleeds13. The present study also provided robust evidence of an association of CMBs with arteriolosclerosis in community-based older adults. Prior autopsy studies in clinical settings with fewer than 11 patients did note that CMBs were often present near arteriosclerotic vessels, which could help to explain the link between CMBs and arteriosclerosis17,18. However, arteriolosclerosis involves thickening of the vessel wall and narrowing of the lumen of arterioles and thus a link between diseased arterioles and CMBs is plausible. Finally, the present work demonstrated that frontal lobe CMBs drive the relationship with CAA and arteriolosclerosis. Previous investigations have shown that CMBs are not colocalized with CAA pathology19. Additional research to investigate the underlying mechanisms for the links to frontal lobe CMBs is warranted.Conclusion
The present study on the neuropathologic correlates of cerebral microbleeds combined detailed neuropathologic examination and ex-vivo MRI in a large number of community-based older adults. It was demonstrated that cerebral microbleeds are associated with CAA and arteriolosclerosis. This was true when considering the total number of microbleeds as well as the number of microbleeds in the frontal lobe.Acknowledgements
National Institute of Neurological Disorders and Stroke (NINDS) UH2-UH3NS100599
National Institute on Aging (NIA) R01AG064233
National Institute on Aging (NIA) R01AG067482
National Institute on Aging (NIA) R01AG017917
National Institute on Aging (NIA) R01AG015819
National Institute on Aging (NIA) P30AG010161
References
1. Poels MM, Vernooij MW, Ikram MA, et al. Prevalence and risk factors of cerebral microbleeds: an update of the Rotterdam scan study. Stroke. 2010;41(10 Suppl):S103-S106.
2. Yubi T, Hata J, Ohara T, et al. Prevalence of and risk factors for cerebral microbleeds in a general Japanese elderly community. Neurol Clin Pract. 2018;8(3):223-231.
3. Wiegman AF, Meier IB, Schupf N, et al. Cerebral microbleeds in a multiethnic elderly community: demographic and clinical correlates. J Neurol Sci. 2014;345(1-2):125-130.
4. Lau KK, Wong YK, Teo KC, et al. Long-Term Prognostic Implications of Cerebral Microbleeds in Chinese Patients With Ischemic Stroke. J Am Heart Assoc. 2017;6(12):e007360. Published 2017 Dec 7.
5. Akoudad S, Wolters FJ, Viswanathan A, et al. Association of Cerebral Microbleeds With Cognitive Decline and Dementia. JAMA Neurol. 2016;73(8):934-943.
6. Ding J, Sigurðsson S, Jónsson PV, et al. Space and location of cerebral microbleeds, cognitive decline, and dementia in the community. Neurology. 2017;88(22):2089-2097.
7. Meier IB, Gu Y, Guzaman VA, et al. Lobar microbleeds are associated with a decline in executive functioning in older adults. Cerebrovasc Dis. 2014;38(5):377-383.
8. Martinez-Ramirez S, Romero JR, Shoamanesh A, et al. Diagnostic value of lobar microbleeds in individuals without intracerebral hemorrhage. Alzheimers Dement. 2015;11(12):1480-1488.
9. Guidoux C, Hauw JJ, Klein IF, et al. Amyloid Angiopathy in Brain Hemorrhage: A Postmortem Neuropathological-Magnetic Resonance Imaging Study. Cerebrovasc Dis. 2018;45(3-4):124-131.
10. Pasi M, Pongpitakmetha T, Charidimou A, et al. Cerebellar Microbleed Distribution Patterns and Cerebral Amyloid Angiopathy [published correction appears in Stroke. 2019 Aug;50(8):e240]. Stroke. 2019;50(7):1727-1733.
11. A. Bennett D, A. Schneider J, S. Buchman A, L. Barnes L, A. Boyle P, S. Wilson R.Overview and Findings from the Rush Memory and Aging Project. Curr Alzheimer Res. 2012;9(6):646-663.
12. A. Bennett D, A. Schneider J, Arvanitakis Z, S. Wilson R. Overview and Findings from the Religious Orders Study. Curr Alzheimer Res. 2012;9(6):628-645.
13. Charidimou A, Boulouis G, Gurol ME, et al. Emerging concepts in sporadic cerebral amyloid angiopathy. Brain. 2017;140(7):1829-1850.
14. Greenberg SM, Al-Shahi Salman R, Biessels GJ, et al. Outcome markers for clinical trials in cerebral amyloid angiopathy. Lancet Neurol. 2014;13(4):419-428.
15. Graff-Radford J, Lesnick T, Rabinstein AA, et al. Cerebral microbleed incidence, relationship to amyloid burden: The Mayo Clinic Study of Aging. Neurology. 2020;94(2):e190-e199.
16. Park JH, Seo SW, Kim C, et al. Pathogenesis of cerebral microbleeds: In vivo imaging of amyloid and subcortical ischemic small vessel disease in 226 individuals with cognitive impairment. Ann Neurol. 2013;73(5):584-593.
17. Tanaka A, Ueno Y, Nakayama Y, Takano K, Takebayashi S. Small chronic hemorrhages and ischemic lesions in association with spontaneous intracerebral hematomas. Stroke. 1999;30(8):1637-1642.
18. Fazekas F, Kleinert R, Roob G, et al. Histopathologic analysis of foci of signal loss on gradient-echo T2*-weighted MR images in patients with spontaneous intracerebral hemorrhage: evidence of microangiopathy-related microbleeds. AJNR Am J Neuroradiol. 1999;20(4):637-642.
19. Kövari E, Charidimou A, Herrmann FR, Giannakopoulos P, Bouras C, Gold G. No neuropathological evidence for a direct topographical relation between microbleeds and cerebral amyloid angiopathy. Acta Neuropathol Commun. 2015;3:49.