4127
Computer-Aided Detection of Lacunes from FLAIR and T1-MPRAGE MR Images via 3D Multi-Scale Residual Networks
Mohammed A. Al-masni1, Woo-Ram Kim2, Eung Yeop Kim3, Young Noh4, and Dong-Hyun Kim1
1Department of Electrical and Electronic Engineering, College of Engineering, Yonsei University, Seoul, Korea, Republic of, 2Neuroscience Research Institute, Gachon University, Incheon, Korea, Republic of, 3Department of Radiology, Gachon University College of Medicine, Gachon University, Incheon, Korea, Republic of, 4Department of Neurology, Gachon University College of Medicine, Gachon University, Incheon, Korea, Republic of
1Department of Electrical and Electronic Engineering, College of Engineering, Yonsei University, Seoul, Korea, Republic of, 2Neuroscience Research Institute, Gachon University, Incheon, Korea, Republic of, 3Department of Radiology, Gachon University College of Medicine, Gachon University, Incheon, Korea, Republic of, 4Department of Neurology, Gachon University College of Medicine, Gachon University, Incheon, Korea, Republic of
Synopsis
Lacunes are small cerebrospinal fluid-filled lesions that are generated by the occlusion of penetrating deep branches of cerebral arteries. Early detection of lacunes could decrease the possible clinical implications such as dementia, gait impairment, and lacunar stroke. In this study, we propose a deep learning 3D multi-scale residual network for lacunes identification using FLAIR and T1-MPRAGE MR images. We redesign the proposed network via applying multiple parallel paths using different input scales. This enables to extract more robust contextual global features and hence achieve better detection performance. The proposed work exhibits its ability to distinguish true lacunes from non-lacunes.
Introduction
Lacunes or Lacunar infarcts are small lesions that result due to the blockage of penetrating branches of the main cerebral arteries. Lacunes have round cavity shapes of less than 15 mm in diameter and located in the basal ganglia or non-cortical white matter 1. They have been considered as diagnostic biomarkers of various cerebrovascular diseases including dementia, gait impairment, and lacunar stroke. Magnetic resonance imaging (MRI) is the preferred scanning tool for lacune screening. More specifically, clinicians usually examine the presence of lacunes using fluid-attenuated inversion recovery (FLAIR) and T1-weighted magnetization prepared rapid gradient echo (T1-MPRAGE). Although these image modalities assist neuroradiologists to improve the identification of lacunes, manual screening via naked eyes is still labor-intensive, subjective, and time-consuming. Thus, the development of a computer-aided detection system for lacune is extremely important through which a second opinion can be provided to neuroradiologists.Methods
The goal of the proposed work is to discriminate between suspicious lacune candidates that have been priorly assigned, leading to provide confidence on the lacune samples by differentiating between them and lacune mimics.[Dataset]
A total of 288 subjects were acquired using two MRI protocols (i.e., FLAIR and T1-MPRAGE), including 696 lacunes. The ground-truth labeling of lacunar infarcts was performed by expert neuroradiologists. In the case of lacune patches generation, we captured 3D multi-scale patches surrounding each labeled lacune at three different scales of 32×32×5, 48×48×5, and 64×64×5 voxels from both FLAIR and T1-MPRAGE images. To increase the number of positive samples (i.e., lacune patches), we augmented every generated 3D patch six times by applying four rotations and two different shifting operations, providing a total of 4,176 samples. For the non-lacune samples, we randomly extracted an equal amount of 4,176 negative samples in order to train the network using a balanced dataset and avoid any bias towards the majority class.
[Proposed 3D ResNet]
In this work, we implemented a 3D version of the ResNet to discriminate between the suspicious lacune and non-lacune candidates. The detailed structure of the proposed 3D ResNet is illustrated in Figure 1. The network consists of two prior convolutional layers followed by three residual blocks, including two different bypass connections. Each residual block starts by adding residual features generated by an additional convolutional layer, while the second connection is performed by attaching the identity mapping of the preceding layer to be added to the main path. All the utilized convolutional layers are with filter sizes of 3×3×3 and feature maps of k1=12, k2=24, k3=36, and k4=48. The proposed network learns global contextual representations from six parallel paths (i.e., three different scales for each imaging modality). Then, we concatenate all the generated features from these six paths and pass them into two fully connected neural network (FCNN) layers with 100 and two neurons, respectively.
Results
The overall evaluation during the training process is demonstrated in Figure 2. This figure shows how the performance accuracy was improved and the loss function was declined throughout the training epochs for both training and validation sets. The results show the capability of the proposed network, trained using three various scales of two different image modalities (i.e., FLAIR and T1-MPRAGE), on the differentiation between lacune and non-lacune candidates. As an overall evaluation through all five-fold tests, the 3D ResNet correctly identifies 4,026/4,176 lacunes and 3,797/4,176 non-lacunes, obtaining sensitivity and specificity of 96.41% and 90.92%, respectively. We also achieve an overall precision of 91.40%, F1-score of 93.84%, and accuracy of 93.67%. Further, we illustrate the ROC curves and their AUCs of the proposed work over the five-fold cross-validation in Figure 3. The proposed work achieves an overall AUC of 93.67% for all fold tests. In addition, Figure 4 presents some lacune and non-lacune exemplars that were truly identified and detected through the proposed 3D multi-scale residual networks. The drawn circles on the brain MR FLAIR and T1-MPRAGE images reflect the locations of suspicious candidates that were tested using the proposed network.Discussion
In this study, the proposed network has the ability to learn multi-scale global features, which is designed as six parallel paths (i.e., three different scales for each imaging modality). Then, the extracted features from all paths are concatenated and passed into the FCNN classifier. The proposed network can promote the neuroradiologists’ decision with a second opinion by providing a supportive interpretation of each potential candidate. The main limitation of this study is that the proposed work is a semi-automated technique, which requires minimal user intervention. A mechanism should exist which first selects the potential suspicious candidates. After that, the network directly learns multi-scale features from these candidates in a supervised manner. However, providing an efficient and fully automated single network to tackle the detection of lacunes can be a complicated and challenging task. Due to this, some researchers attempted to solve this issue by providing two-cascaded approaches 2-5, where the first stage was responsible to screen and detect potential candidates, while the second stage intended to discriminate the remaining candidates.Conclusion
The proposed work could be feasible for clinical usage by providing a supportive decision towards lacunar infarcts detection.Acknowledgements
This research was supported by the Brain Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (2018M3C7A1056884). This work was also supported by a grant of the Korea Healthcare Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant No: HI14C1135).References
- Wardlaw, J. M. et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol 12, 822-838, doi:Doi 10.1016/S1474-4422(13)70124-8 (2013).
- Ghafoorian, M. et al. Deep multi-scale location-aware 3D convolutional neural networks for automated detection of lacunes of presumed vascular origin. Neuroimage-Clin 14, 391-399, doi:10.1016/j.nicl.2017.01.033 (2017).
- Dou, Q. et al. Automatic Detection of Cerebral Microbleeds From MR Images via 3D Convolutional Neural Networks. IEEE Transactions on Medical Imaging 35, 1182-1195, doi:10.1109/Tmi.2016.2528129 (2016).
- Al-masni, M. A., Kim, W.-R., Kim, E. Y., YoungNoh & Dong-HyunKim. Automated detection of cerebral microbleeds in MR images: A two-stage deep learning approach. NeuroImage: Clinical 28, 102464 (2020).
- Liu, S. F. et al. Cerebral microbleed detection using Susceptibility Weighted Imaging and deep learning. Neuroimage 198, 271-282, doi:10.1016/j.neuroimage.2019.05.046 (2019).
Figures
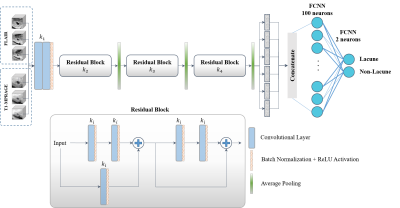
Figure 1. A detailed structure of the proposed 3D
multi-scale ResNet.
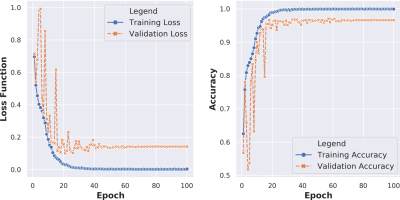
Figure 2. Training
and validation losses (left) and accuracies (right) throughout the network
training over 100 epochs.
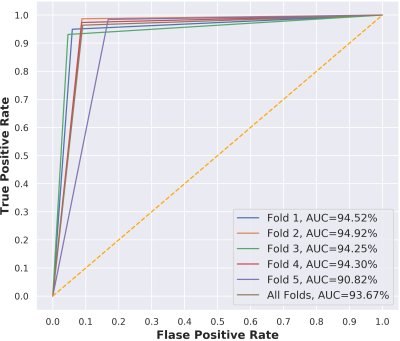
Figure 3. ROC curves with their
AUCs of the proposed 3D ResNet throughout the five-fold cross-validation.
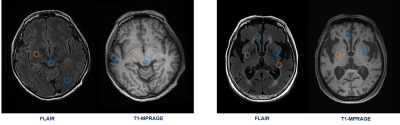
Figure 4. Some exemplar results
of the proposed 3D multi-scale ResNet. The drawn orange and blue circles refer
to the truly detected lacune and non-lacune lesions, respectively.