4117
Rapid submillimeter high-resolution prostate T2 mapping with a deep learning constrained Compressed SENSE reconstruction1Philips Japan, Tokyo, Japan, 2Radiology, Tokyo Metropolitan Police Hospital, Tokyo, Japan, 3Philips Healthcare, Best, Netherlands
Synopsis
Compressed SENSE-AI, based on Adaptive-CS-Net, clearly reduces noise artifacts and significantly improves the accuracy and robustness of T2 values in submillimeter (0.7mm) high-resolution prostate multi-echo turbo spin-echo T2 mapping compared with conventional SENSE and Compressed SENSE techniques, without any penalty for scan parameters. This technique may prove of value in better discriminating prostate cancer from healthy tissues.
PURPOSE
Quantitative MRI is promising for assessing tumor aggressiveness1. One quantitative MR parameter, T2 relaxation time, reflects the interaction of water molecules on a cellular level and is therefore potentially highly sensitive to detect pathological tissue alterations and could be a significant predictor of aggressive prostate tumors2-5. In fact, the implementation of T2 mapping is practically challenging because current T2 mapping requires long scan time otherwise lower spatial resolution, it is unacceptable to be added in routine examination and thus far limited to research settings. One study demonstrated the usefulness of 2D multi-slice, multi-echo TSE (ME-TSE) T2 mapping sequence combined with Compressed SENSE (C-SENSE) with higher (=6) reduction factor6. This is a promising method, but the actual in-plane spatial resolution is still 2 mm, it is too far from routine T2 weighted images7.Recently, integrating artificial intelligence (AI) into the MRI reconstruction has attracted much attention to further accelerate scans. At 2019 fastMRI challenge, a novel deep neural network was introduced as Adaptive-CS-Net and showed superior performance for reconstructing the knee image from highly undersampled k-space data8-11. The Adaptive-CS-Net was expanded to multiple contrasts and anatomical areas and presented here as a Compressed SENSE AI (CS-AI) reconstruction.
We attempt to utilize the CS-AI framework for further increasing the in-plane spatial resolution to less than 1 mm in ME-TSE T2 mapping. The purpose of this study is to demonstrate the usefulness of CS-AI in submilimeter high-resolution prostate T2 mapping in volunteers, compared to conventional T2 mapping with SENSE or C-SENSE.
METHODS
A total of five volunteers were examined on a 3.0T system (Ingenia Elition X, Philips Healthcare). The study was approved by the local IRB, and written informed consent was obtained from all subjects.T2 maps and individual echo images of CS-AI were compared with those originating from SENSE and C-SENESE images for image quality, especially for the reduction of image noise. To demonstrate the improvement of T2 values by the effect of CS-AI noise reduction, we compared T2 values with SENSE, C-SENSE and CS-AI scans with the same scan parameters. ROIs were placed on left and right transition zones (TZ) and left and right peripheral zones (PZ). The average T2 values of TZ and PZ were used for comparison among SENSE, C-SENSE and CS-AI. We also compared with the T2 values reported in literature5. Respective T2 values were assessed by using one-way repeated-measures analysis of variance (ANOVA) and post hoc Tukey test.
Imaging parameters for ME-TSE T2mapping were: voxel size=0.7*0.7*3mm3, FOV=200*200mm, 20slices, TR=4000ms, TE=20ms*8 echoes, TSE factor=8, SENSE/C-SENSE acceleration factor=12, and total acquisition time=3min40s. SENSE/C-SENSE factor in right-left direction was chosen to minimize the respiratory motion artifacts.
RESULTS and DISCUSSION
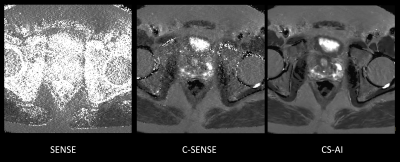
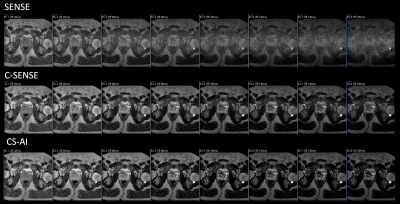
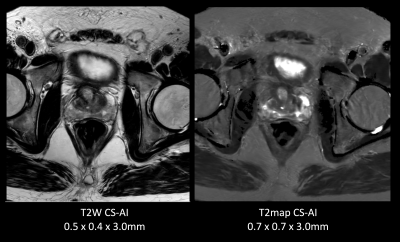
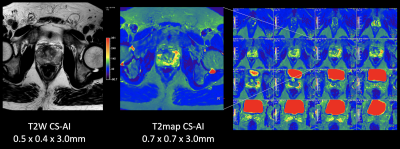
Figure 1 and 2 shows representative T2 map [Fig.1] and each echo source images [Fig.2] of ME-TSE T2 mapping with SENSE, C-SENSE and CS-AI. CS-AI most clearly reduced the noise which exists in the center of the SENSE and C-SENSE images particularly in later echoes, resulting in the best T2 map image quality. Comparison of high-resolution T2 weighted image and T2 map with CS-AI is shown in Figure 3 and representative high-resolution color T2 maps using CS-AI are shown in Figure 4. CS-AI T2 map with 0.7 mm2 in-plane resolution showed comparable morphological information with T2W images. The T2 values with CS-AI were close to the values reported in literature2,3 with smallest standard deviation, whereas the values with SENSE/C-SENSE were higher, most likely due to the presence of severe noise over the prostate. This suggests that CS-AI might provide more accurate T2 values in submillimeter high-resolution images with high robustness. Although further clinical investigation is needed, high-resolution T2 mapping with CS-AI might be clinically useful in assessment of prostate cancer in more detail.CONCLUSION
CS-AI clearly reduces noise artifacts and significantly improves the accuracy and robustness of T2 values in submillimeter high-resolution prostate ME-TSE T2 mapping compared with conventional SENSE and C-SENSE techniques, without any penalty for scan parameters. This technique may prove of value in better discriminating prostate cancer from healthy tissues.Acknowledgements
No acknowledgement found.References
1. Langer DL, et al. Prostate cancer detection with multi-parametric MRI: logistic regression analysis of quantitative T2, diffusion-weighted imaging, and dynamic contrast-enhanced MRI. Magn Reson Imaging. 2009 Aug;30(2):327-34.
2. Hoang Dinh A, et al. Characterization of prostate cancer using T2 mapping at 3T: a multi-scanner study. Diagn Interv Imaging. 2015 Apr;96(4):365-72.
3. Liu W, et al. Accelerated T2 mapping for characterization of prostate cancer. Magn Reson Med. 2011 May;65(5):1400-6.
4. van Houdt PJ, et al. Performance of a fast and high‐resolution multi‐echo spin‐echo sequence for prostate T2 mapping across multiple systems. Magn Reson Med 2018; 79: 1586‐1594.
5. Lee CH. Quantitative T2‐mapping using MRI for detection of prostate malignancy: A systematic review of the literature. Acta Radiol 2019; 60: 1181‐1189.
6. Noda S, et al. A study on Compressed SENSE(CSENSE) in prostate T2map at 3T. Proc Intl Soc Mag Reson Med. 2018;26:4505.
7. Weinreb JC, et al. PI-RADS prostate imaging–reporting and data system: 2015, version 2. European Urology 2016;69:16-40.
8. Knoll F, Zbontar J, Sriram A, et al. fastMRI: A Publicly Available Raw k-Space and DICOM Dataset of Knee Images for Accelerated MR Image Reconstruction Using Machine Learning. Radiol Artif Intell. 2020;2(1):e190007.
9. Knoll F, et al. Advancing machine learning for MR image reconstruction with an open competition: Overview of the 2019 fastMRI challenge. Magn Reson Med. 2020;(January):mrm.28338.
10. Pezzotti N, et al. An Adaptive Intelligence Algorithm for Undersampled Knee MRI Reconstruction. IEEE Access. 2020;8:204825-204838.
11. Pezzotti N, et al. Adaptive-CS-Net: FastMRI with Adaptive Intelligence. arxiv. 2019;(NeurIPS).12. Yoneyama, et al. Noise Reduction in Prostate Single-Shot DW-EPI utilizing Compressed SENSE Framework. Proc Intl Soc Mag Reson Med. 2019;27:1634
Figures