4096
Application of High Spectral and Spatial resolution (HiSS) MRI in prostate: a pilot study1Radiology, The University of Chicago, Chicago, IL, United States, 2Sanford J. Grossman Center of Excellence in Prostate Imaging and Image Guided Therapy, The University of Chicago, Chicago, IL, United States, 3Philips Research NA, Cambridge, MA, United States, 4Pathology, The University of Chicago, Chicago, IL, United States
Synopsis
HiSS MRI is an echo-planar spectroscopic imaging (EPSI)-based method focusing on the shape and structure of the water and fat resonances. In the prostate, temporal signal decay parameters (ROC AUC 0.74-0.91) and spectral resonance shape parameters (ROC AUC 0.83-0.91) show high diagnostic potential. Spectral shape parameters do not correlate strongly with temporal decay parameters. Thus, they carry information complementary to the standard clinical multi-parametric MRI and can likely be utilized to improve its diagnostic performance in prostate cancer.
INTRODUCTION
High Spectral and Spatial resolution (HiSS) MRI is an echo-planar spectroscopic imaging (EPSI)-based method that focuses on the shape and structure of the water and fat resonances. It acquires coherent multi-echo images, hence providing spectral information, and has been shown to have high diagnostic potential in breast cancer.1-3 The less favorable coil geometry and histological differences make it unclear whether HiSS MRI can be translated to prostate imaging, though. Here, we evaluate the feasibility and diagnostic potential of HiSS MRI in prostate cancer.METHODS
Six patients scheduled for prostatectomy underwent prostate MRI without an endorectal coil on a 3T Philips Ingenia scanner. The MRI protocol included T2-weigthed MRI (T2w), DWI, DCEMRI, and HiSS MRI (in-plane resolution 0.8-1.25 mm, three 3.0 mm slices, 0.5 mm gap, spectral resolution 2.6-3.5 Hz, TR/TE 463/230 - 652/162 ms, flip angle 90 deg, SENSE factor 1.5), with HiSS MRI acquired after contrast injection. DWI images were processed to obtain apparent diffusion coefficient (ADC) maps. The 1 cm HiSS MRI slab was centered on the largest lesion seen on T2w and yielded a train of 127 phase-coherent gradient echo images. The echo train was Fourier transformed yielding the proton spectrum in each voxel, revealing water resonance detail. In the temporal domain, changes in logarithm of voxel signal intensity were analyzed and linear (R) and quadratic (R1, R2) quantifiers of decay were calculated.4 In the spectral domain, three parameters independent on signal scaling were calculated: water resonance peak width (PW), relative peak asymmetry (PRA), and relative peak distortion from ideal Lorentzian shape (PRD).5-8 MRI images were correlated with prostatectomy to outline 7 cancer and 5 benign regions of interest (ROIs). The 6 HiSS MRI-derived parameters (R, R1, R2, PW, PRA, PRD) were calculated on a voxel-by-voxel basis, and analysis was conducted on their ROI averages. Differences between values calculated in cancerous and benign ROIs were evaluated using the one-sided t-test. Correlation between parameters was evaluated using the Spearman correlation coefficient. The statistical significance level was set to 0.05. Diagnostic performance was evaluated via ROC analysis and quantified with ROC AUC.RESULTS
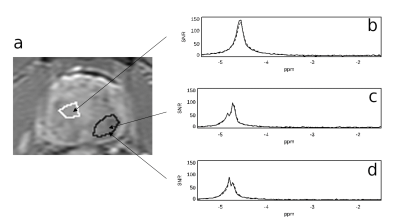
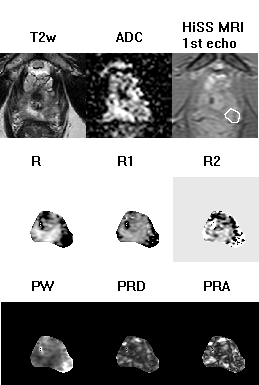
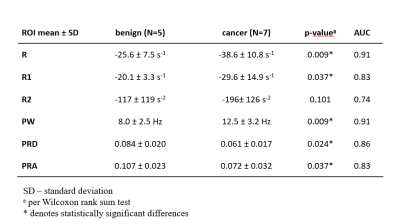
Figure 1 shows representative spectra in three voxels in an axial slice through a prostate, demonstrating high signal to noise ratio (SNR, typically 50-150 in a single voxel) and high reproducibility of water resonance shape and/or structure on repeated measurements. Figure 2 depicts the T2w image, ADC map, shortest-TE HiSS MRI image, and maps of 6 HiSS MRI-derived parameters, for a representative case.The statistics, p-values, and receiver operator characteristic area under the curve (ROC AUC) values for the HiSS MRI parameters are shown in Table 1. Spectral domain quantifiers performed better overall than temporal domain quantifiers, with the highest ROC AUC values found in R (0.91) and PW (0.91). All HiSS MRI-derived parameters showed statistically significant differences between benign and cancerous ROI values, except for R2. PRD and PRA increase with increased resonance shape distortion and are thus markers of tissue inhomogeneity, and were relatively decreased in cancer ROIs.
The Spearman correlation coefficient was high between PW and R (-0.95), which are both inversely related to T2* values, and between PRD and PRA (0.84), which are both markers of a non-Lorentzian water resonance shape. The correlation coefficients between PW and R, one one hand, and PRD and PRA, on the other, were low (-0.25 - +0.38).
DISCUSSION
High SNR and reproducibility of HiSS MRI-acquired spectra demonstrate feasibility of HiSS MRI in the prostate at 3T without an endorectal coil. The high SNR indicates the possibility of whole-prostate coverage in clinically feasible times using increased acceleration factors. The cancer and normal tissue ROIs were drawn on the T1-weighted, shortest-TE HiSS MRI image, and thus were effectively blinded to HiSS contrast. Further, the ROIs were not paired and the parameter values were not normalized in an intra-subject manner. Thus, HiSS MRI-derived parameters reported here are absolute quantifiers that can support inter-subject comparisons.The spectral domain parameters showed better overall diagnostic performance than temporal domain parameters. This is likely due to the fact that the spectral parameters utilize the phase coherence along the echo train, and thus have higher information content. Interestingly, PRD and PRA are reduced in prostate cancer ROIs. This is opposite to breast imaging results, where cancer is marked with increased heterogeneity, but is consistent with the higher tissue homogeneity in epithelium-dense prostate cancer.
Low absolute values of the coefficients of correlation between PW and R (characterizing peak broadening and hence the temporal decay constant) on one hand, and PRD and PRA (characterizing peak shape and structure) on the other, are notable. They indicate that PRD and PRA carry information complementary to the standard clinical mp-MRI and could thus be leveraged to improve accuracy.
CONCLUSION
Our results demonstrate the feasibility of HiSS MRI in the prostate at 3T without an endorectal coil and its potential utility for diagnosis of prostate cancer. Spectral domain parameters showed better diagnostic performance overall, likely due to the higher information content. Water resonance structure quantifiers are complementary to the standard multi-parametric MRI of the prostate and thus have potential to improve diagnostic accuracy.Acknowledgements
This work was supported by the University of Chicago Comprehensive Cancer Center from the National Cancer Institute Cancer Center Support Grant P30CA014599; the National Institutes of Health (NIH 5R01CA167785, NIH 1R01CA228036-01A1, R01CA172801; and S10OD018448); Philips Research, NA; Guerbet LLC; and the Segal Foundation.References
1. Medved M, Fan X, Abe H, et al. Non-contrast enhanced MRI for evaluation of breast lesions: comparison of non-contrast enhanced high spectral and spatial resolution (HiSS) images versus contrast enhanced fat-suppressed images. Acad Radiol. 2011;18(12):1467-1474.
2. Medved M, Ivancevic MK, Olopade OI, Newstead GM, Karczmar GS. Echo-planar spectroscopic imaging (EPSI) of the water resonance structure in human breast using sensitivity encoding (SENSE). Magn Reson Med. 2010;63(6):1557-1563.
3. Medved M, Li H, Abe H, et al. Fast bilateral breast coverage with high spectral and spatial resolution (HiSS) MRI at 3T. J Magn Reson Imaging. 2017;46(5):1341-1348.
4. Ciris PA, Balasubramanian M, Seethamraju RT, et al. Characterization of gradient echo signal decays in healthy and cancerous prostate at 3T improves with a Gaussian augmentation of the mono-exponential (GAME) model. NMR Biomed. 2016;29(7):999-1009.
5. Foxley S, Fan X, Mustafi D, et al. Quantitative analysis of water proton spectral lineshape: a novel source of contrast in MRI. Phys Med Biol. 2008;53(17):4509-4522.
6. Medved M, Newstead GM, Fan X, et al. Fourier component imaging of water resonance in the human breast provides markers for malignancy. Phys Med Biol. 2009;54(19):5767-5779.
7. Wood AM, Medved M, Bacchus ID, et al. Classification of breast lesions pre-contrast injection using water resonance lineshape analysis. NMR Biomed. 2013;26(5):569-577.
8. Weiss WA, Medved M, Karczmar GS, Giger ML. Residual analysis of the water resonance signal in breast lesions imaged with high spectral and spatial resolution (HiSS) MRI: a pilot study. Med Phys. 2014;41(1):012303.
Figures