4085
Directional and Inter-acquisition Variability in DWI (DAVID)1Radiology, The University of Chicago, Chicago, IL, United States, 2Sanford J. Grossman Center of Excellence in Prostate Imaging and Image Guided Therapy, The University of Chicago, Chicago, IL, United States, 3Research Computing Center, The University of Chicago, Chicago, IL, United States, 4Department of Public Health Sciences, The University of Chicago, Chicago, IL, United States, 5Philips Research North America, Cambridge, MA, United States
Synopsis
Diffusion Weighted Imaging (DWI) MRI detects prostate cancers but is very sensitive to motion artifacts. There has been little quantitative evaluation of variability to guide clinical use of DWI. We found very high variability between individual acquisitions used for averaging at high b-values (% ranges of 74.08% - 115.56% in cancers and 53.53% - 159.91% in normal prostate tissue), primarily due to motion during diffusion-sensitizing gradients. High signals in cancer voxels appear in some acquisitions but not others. Therefore, standard averaging can obscure cancers. We propose alternative methods for combining information from individual images at each b-value to maximize diagnostic accuracy.
Introduction
Diffusion Weighted Imaging (DWI) is an essential part of multi-parametric MRI (mpMRI) for the detection of prostate cancers.1,2 However, it is well understood that DWI is sensitive to motion artifacts. Small motions during the application of diffusion-sensitizing gradients can result in significant loss of signal.3 This signal loss is especially problematic for high b-value scans. These scans have low signal-to-noise ratio and require multiple acquisitions for each of 3 diffusion-sensitizing gradient directions (X,Y,Z).4 In clinical practice, these acquisitions are combined using standard averaging to produce a final composite image that is analyzed by radiologists. There is little quantitative information regarding directional and inter-acquisition variability in DWI (DAVID) of the prostate. Here, we analyze signal variability between the individual acquisitions at high b-values that are used to create composite diffusion-weighted images. We show that, due to the nature of the motion artifacts, standard averaging is not optimal for combining the acquisitions. In fact, standard averaging can result in significant loss of valuable diagnostic information. We propose an alternative method for combining acquisitions for each diffusion-sensitizing gradient direction.Materials and Methods
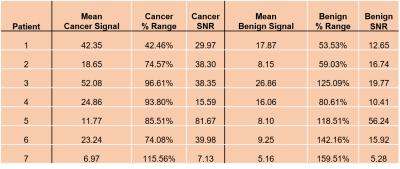
Diffusion-weighted images were acquired on a 3T Philips Ingenia with 3 diffusion-sensitizing gradients and 4-8 acquisitions per gradient direction at high b-values of either 900 or 1500 sec/mm2. Four patients (Patients 1-4) were imaged with an endorectal coil and three patients were imaged without an endorectal coil (Patients 5-7). Raw k-space data was exported from scanners and reconstructed using Reconframe (Gyrotools, Zurich, Switzerland). For analysis, only one cancer ROI selected by a radiologist (based on PI-RADSv2.1) was analyzed per patient.Signal standard deviation (SD) and signal-to-noise ratio (SNR), SNR = signal/root-mean-square noise, were calculated. % Range of the average mean was calculated from:
$$\text{% Range} = \frac{\text{Average Range}}{\text{Average Mean}} *100$$
where Average Range is the average of the ranges calculated per voxel (3x3 voxels in cancer tissue) across all acquisitions and Average Mean is the average of the mean signals per voxel (3x3 voxels in benign tissue) across all acquisitions.
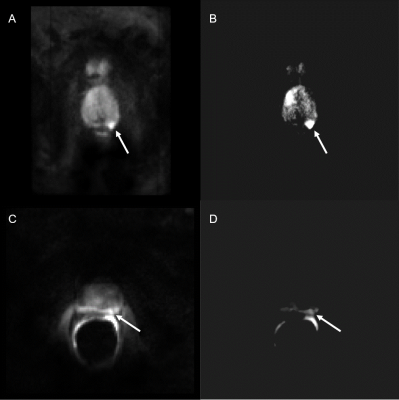
“Editing for Restricted Diffusion” (ERD) is based on the hypothesis that high intensity in each voxel is due to restricted diffusion, while signals well below the maximum (over acquisitions) for each voxel are likely artifacts due to motion. For each voxel in each series of acquisitions at each b value, we discarded all voxel values that were less than 75% of the maximum for that voxel across all acquisitions. The remaining signals were then summed to produce the final image. Thus, the voxel intensity in the final ERD image emphasized the highest intensities detected in each voxel.
Results
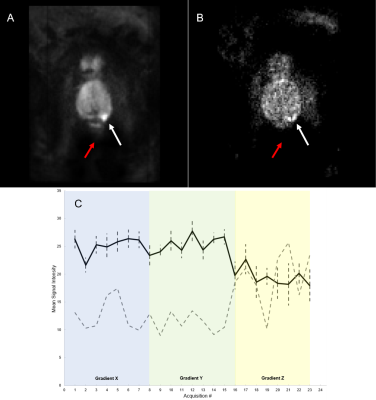
Voxel-by-voxel measurements of SD reveal large signal variation between independent acquisitions for each diffusion-encoding-gradient direction, ‘X’,’Y’, and ‘Z’. Figure 1 (B and C) shows significant signal variation throughout the prostate and especially in the cancer ROI. The noise level, indicated by the red arrow in the figure for patient 6, is much lower than the variation in signal (SNR = 40). For patient 6, signal acquired with diffusion-sensitizing gradient in the ‘Z’ direction is lower and has higher variation vs. other directions (t-test comparing mean signals of Direction X to Z (p<0.01) and Direction Y to Z (p<0.01)). There were statistically significant differences between signal intensities for the different directions and acquisitions in the other patients. Variability between acquisitions was significantly higher than the noise level for all patients studied, both in the cancer ROI (74.08% - 115.56 % range) and the benign contralateral tissue (53.53% - 159.91% range) (Table 1). Registration of the individual images using Demons deformable registration did not decrease variability. As shown in Figure 2, editing signals to emphasize restricted diffusion, ‘ERD’, emphasizes the high signals in cancers when images are acquired both without (Figure 2B) and with (Figure 2D) the endorectal coil.Discussion
The variation in the prostate signal between acquisitions is much greater than the noise level and is probably due to motion artifacts. Since deformable registration did not reduce variability, we conclude that most of the variability is due to motion during application of diffusion-sensitizing gradients. Regions with restricted diffusion are particularly sensitive to these motion artifacts. At high b-value, signals from parts of cancers appear in some images but not in others. Averaging the signals that show the cancers together with signals that do not show the cancers can reduce cancer conspicuity and produce errors in measurements of restricted diffusion. The results demonstrate that ERD is a viable method of selecting and preserving cancer signals across acquisitions. This approach assumes that high intensity signals at high b-value acquisitions are due to restricted diffusion, e.g. in cancers. This introduces bias. Some high intensity signals can be due to artifacts, such as signal pile-up, T2 shine-through and coil artifacts. As a result, ERD can introduce false positives. More sophisticated approaches to ERD will involve the use of cluster analysis and AI to identify suspicious tissues and exclude artifacts.Conclusion
The standard approach to averaging individual diffusion-weighted acquisitions may obscure signals from cancers. Alternatives such as ERD are needed to ensure that cancers are reliably diagnosed with DWI.Acknowledgements
This research is supported by National Institutes of Health (R01 CA172801, R01CA218700, 1S10OD018448-01), University of Chicago Comprehensive Cancer Center Support Grant (Grant No. P30CA014599), and the Sanford J Grossman Charitable Trust.References
1. Johnson LM, Turkbey B, Figg WD, Choyke PL. Multiparametric MRI in prostate cancer management. Nature Reviews Clinical Oncology. 2014;11(6):346-353.
2. Kim CK, Park BK, Kim B. High-b-Value Diffusion-Weighted Imaging at 3 T to Detect Prostate Cancer: Comparisons Between b values of 1,000 and 2,000 s/mm2. American Journal of Roentgenology. 2010;194(1):W33-W37.
3. Le Bihan D, Poupon C, Amadon A, Lethimonnier F. Artifacts and Pitfalls in Diffusion MRI. Journal of Magnetic Resonance Imaging. 2006;24(3):478-488.
4. Kitajima K, Kaji Y, Kuroda K, Sugimura K. High b-value Diffusion-weighted Imaging in Normal and Malignant Peripherizal Zone Tissue of the Prostate: Effect of Signal-to-noise ratio. Magnetic Resonance in Medical Sciences. 2008;7(2):93-99.
Figures