4071
Impact of sustained Synovitis on Knee Joint Structural Degeneration: 4-Year MRI Data from the Osteoarthritis Initiative1Department of Radiology and Biomedical Imaging, University of California, San Francisco, San Francisco, CA, United States, 2Department of Radiology, Faculty of Medicine, Chiang Mai University, Thailand, Chiang Mai, Thailand, 3Department of Epidemiology and Biostatistics, University of California, San Francisco, San Francisco, CA, United States
Synopsis
Synovial inflammation is a well-known risk factor of osteoarthritis (OA). Several semi-quantitative scores have been developed to grade synovitis using non-contrast enhanced MRI studies of the knee. However, the role of inflammatory processes in the progression of knee OA is yet to be fully understood. One hundred and eighty individuals with right knee MRIs at baseline, 2 and 4 years were studied using MRI-based semi-quantitative synovitis scores measuring presence/absence as well as severity of synovitis Subjects with sustained synovitis had greater progression of meniscus, bone marrow and cartilage abnormalities compared to controls without sustained synovitis.
Summary of main findings
Subjects with sustained synovitis over 4 years had significantly greater progression of meniscus, bone marrow and cartilage degeneration measured using semi-quantitative whole-organ magnetic resonance imaging scores (WORMS) compared to controls without synovitis.Background
Osteoarthritis (OA) is one of leading causes of disability in elderly. 1 One of the well-known risk factors of OA is synovial inflammation. 2,3 Recently, several semi-quantitative scores have been developed to grade synovitis on non-contrast enhanced MRI studies of the knee inflammatory processes that include joint effusion, synovial proliferation and signal changes in Hoffa’s fat pad. To date, there is limited information on how these inflammatory processes impact progression of knee OA. 4-7 The goal of this project was therefore to investigate how sustained synovitis assessed with semi-quantitative MRI impacts knee joint degenerative changes over 4 years.Methods
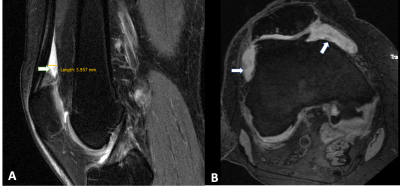
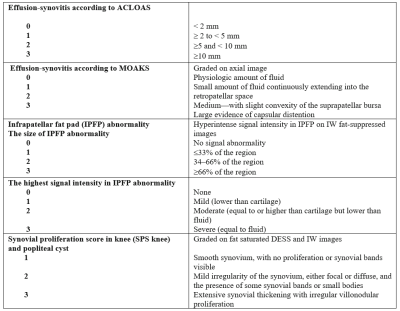
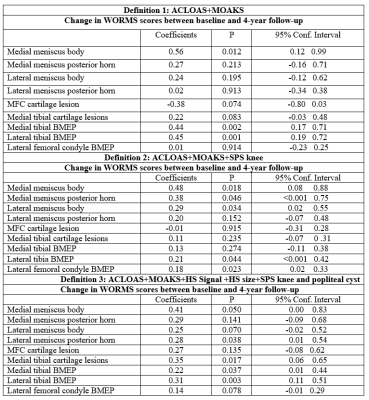
One hundred and eighty individuals from the Osteoarthritis Initiative (OAI) Cohort were selected for this study. Inclusion criteria was radiographic Kellgren-Lawrence (KL) grades 0–3 at baseline, as well as availability of right knee MRIs (with coronal and sagittal FSE sequences) at baseline, 2 and 4 year follow-ups. Exclusion criteria were KL grade 4 knees, knee injury with deformity of the knee joint, total joint replacement at the lower extremities, MRI evidence of fractures or abnormalities, that did not fit into the spectrum of OA such as tumor or inflammation and subjects with a history of inflammatory arthritis diagnosed during follow-up as well as arthroscopy for meniscectomy and ligament repair. Synovitis was scored semi-quantitatively from 0-3 in all of the 3 MRI studies using the Anterior Cruciate Ligament Osteoarthritis Score (ACLOAS), the MRI Osteoarthritis Knee Score (MOAKS), the synovial proliferation score (SPS) at the knee and in a popliteal cyst as well as the size and signal intensity of infrapatellar fat pad (IPFP) synovitis (Table 1, Figure 1 A&B). Three MRI-based definitions of synovitis were used: (i) score >2 based on two variables from MOAKS and ACLOAS; (ii) score > 3 based on three variables from ACLOAS, MOAKS and SPS knee and (iii) score > 6 based on 6 parameters from ACLOAS, MOAKS, SPS knee and popliteal cyst as well as the size and signal of IPFP abnormalities. Absence of synovitis was defined by scores lower than the ones listed above for each of the 3 definitions. Subjects with synovitis (cases) had sustained synovitis scores at baseline, second and fourth year, while subjects without synovitis (controls) had no synovitis at all 3 timepoints. Outcome measures were semi-quantitative gradings of structural abnormalities of cartilage, meniscus and bone marrow assessed with the whole-organ magnetic resonance imaging score (WORMS) between baseline and 4 years. The parameters for WORMS were meniscal lesions in each of the six regions (medial/lateral and anterior/body/posterior) with grades from 0 to 4; bone marrow edema pattern (BMEP) graded from 0-3, and cartilage defects graded from 0 to 6. Differences in outcome measures between the cases and controls were assessed using linear regression models adjusted for age, gender, race, and body mass index.Results
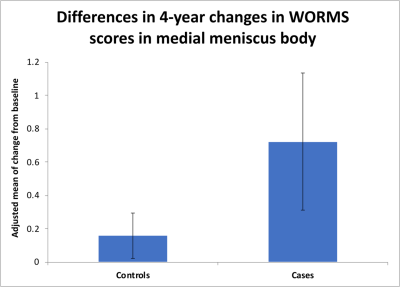
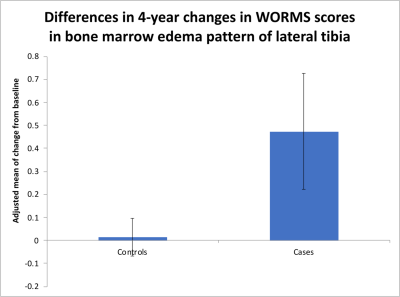
Our results showed that WORMS gradings progressed more in subjects with sustained synovitis compared to controls as shown in Table 2. Findings were statistically significant for the meniscus, bone marrow and cartilage abnormalities. For synovitis definition (i), significant changes were found for anterior horn and body of medial meniscus (Figure 2), and BMEP in medial and lateral tibia (Figure 3). Using definition (ii), posterior horn and body of medial meniscus, body of lateral meniscus, and BMEP in trochlea, lateral femoral condyle and lateral tibia and using definition (iii) body of medial meniscus, posterior horn of lateral meniscus, cartilage in medial tibia, and BMEP of medial and lateral tibia showed significant changes (Table 2).Discussion
Our study found that progression of structural change based on WORMS scores were more often observed in patients with sustained synovitis quantified based on MRI findings. These findings suggest that synovitis is a predictive biomarker of joint damage using knee structural outcomes. 3,8,9 Cartilage degeneration can be promoted by synovial inflammation due secretion of catabolic and pro-inflammatory mediators. 9Conclusion
Our study shows that sustained synovitis at the knee joint is associated with accelerated longitudinal joint degeneration assessed with semi-quantitative MRI biomarkers.Acknowledgements
The OAI is a public-private partnership comprised of five contracts (N01-AR-2-2258; N01-AR-2-2259; N01-AR-2-2260; N01-AR-2-2261; N01-AR-2-2262) funded by the National Institutes of Health, a branch of the Department of Health and Human Services and conducted by the OAI Study Investigators. Private funding partners include Pfizer, Inc.; Novartis Pharmaceuticals Corporation; Merck Research Laboratories; and GlaxoSmithKline. Private sector funding for the OAI is managed by the Foundation for the National Institutes of Health. The analyses in this study were funded through the NIH (National Institute of Arthritis and Musculoskeletal and Skin Diseases grants R01AR064771). We would like to thank the faculty and staff of the Coordinating Center of the OAI at the NIH and UCSF for their invaluable assistance with patient selection, statistical analysis, and technical support.References
1. Guccione AA FD, Anderson JJ, Anthony JM, Zhang Y, Wilson PW, Kelly-Hayes M, Wolf PA, Kreger BE, Kannel WB. . The effects of specific medical conditions on the functional limitations of elders in the Framingham Study. . American journal of public health. 1994 Mar;84;((3):):351-358.
2. Dell'Isola A, Allan R, Smith SL, Marreiros SS, Steultjens M. Identification of clinical phenotypes in knee osteoarthritis: a systematic review of the literature. BMC Musculoskelet Disord. 2016;17(1):425.
3. Wang X, Hunter DJ, Jin X, Ding C. The importance of synovial inflammation in osteoarthritis: current evidence from imaging assessments and clinical trials. Osteoarthritis Cartilage. 2018;26(2):165-174.
4. Wang X, Blizzard L, Halliday A, et al. Association between MRI-detected knee joint regional effusion-synovitis and structural changes in older adults: a cohort study. Ann Rheum Dis. 2016;75(3):519-525.
5. Han W, Aitken D, Zhu Z, et al. Signal intensity alteration in the infrapatellar fat pad at baseline for the prediction of knee symptoms and structure in older adults: a cohort study. Ann Rheum Dis. 2016;75(10):1783-1788.
6. Roemer FW, Guermazi A, Zhang Y, et al. Hoffa's Fat Pad: Evaluation on Unenhanced MR Images as a Measure of Patellofemoral Synovitis in Osteoarthritis. AJR Am J Roentgenol. 2009;192(6):1696-1700.
7. Hill CL, Hunter DJ, Niu J, et al. Synovitis detected on magnetic resonance imaging and its relation to pain and cartilage loss in knee osteoarthritis. Ann Rheum Dis. 2007;66(12):1599-1603.
8. Berenbaum F. Osteoarthritis as an inflammatory disease (osteoarthritis is not osteoarthrosis!). Osteoarthritis Cartilage. 2013;21(1):16-21.
9. Hunter DJ, Zhang W, Conaghan PG, et al. Systematic review of the concurrent and predictive validity of MRI biomarkers in OA. Osteoarthritis Cartilage. 2011;19(5):557-588.
Figures