4062
Identification of Bone Marrow Lesions on Magnetic Resonance Imaging with Weakly Supervised Deep Learning1Department of Medical Imaging, The Third Affiliated Hospital of Southern Medical University, Guangzhou, China, 2College of Information Science and Electronic Engineering, Zhejiang University, Hangzhou, China, 3China International Center, Philips Healthcare, Guangzhou, China, 4Department of Computer Science & Engineering, The Chinese University of Hong Kong, Hong Kong, China
Synopsis
The presence of a bone marrow lesion is associated with incident and progressive knee osteoarthritis (KOA) and joint replacement. Since the ill-defined boundary and various signal strength, identification of bone marrow lesions (BMLs) requires professional diagnostic ability and is subjective. Therefore, we utilize a model to assess whether there exists BMLs in every subregion and their severity on 3D-dual echo steady state (DESS) images according to MRI Osteoarthritis Knee Score (MOAKS). The initiatory results showed that deep learning framework performed well on discrimination of BMLs with good reproducibility.
Purpose and Introduction
KOA is a common musculoskeletal disease which causes limitation of knee function and increases public health and financial burden. In recent years, many studies agree with the opinion that the presence of bone marrow lesions (BMLs) is associated with incident and progression of knee OA1, and it is one of the strongest independent predictors of TKR2. Many semi-quantitative assessments for knee OA have been published to measure relevant lesions in recent years. MRI Osteoarthritis Knee Score (MOAKS) is one of the most reliable and widely used tools assessing several related structures including BMLs. However, it costs lots of time when separating subregions and scoring. Deep learning algorithms, a data-driven machine learning method with convolution neural networks, became widely used for image classification tasks. Therefore, we developed a new deep learning-based scoring model and dichotomous supervised model labeled with MOAKS score to automatically differentiate knee from normal to abnormal, then determine its severity preliminarily on sagittal three-dimensional dual echo steady state (DESS) images from Osteoarthritis Initiate (OAI).Materials and Methods
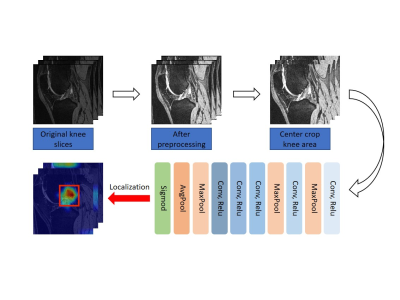
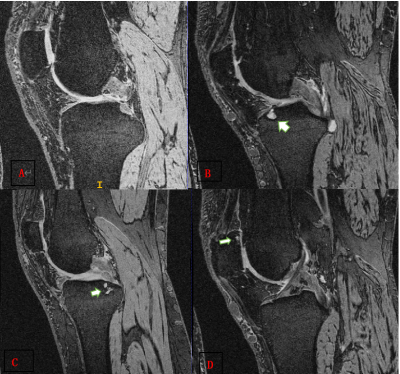
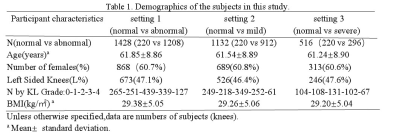
In this retrospective study, based on BMLs MOAKS scores, 1428 subjects underwent MRI examination from the Osteoarthritis Initiative at baseline cohort were defined as followed: 1) normal bone marrow, score 0 for all subregions, 2) abnormal bone marrow, score≥1 for at least one subregion, 3) mild BMLs, score<3 for all subregions and score ≥1 for at least one subregion, 4) severe BMLs, score≥3 for at least one subregion. Then we build three dichotomous data sets indicating the presence or absence of BMLs and severity of lesions. We split the whole dataset into 80% cases for training and 20% cases for testing. The demographics of the participants can be showed in Table 1 and the different severity of BMLs are demonstrated in Figure 3. The deep learning model is learned only with patient-level labels in a weakly supervised way, i.e., without labelling the specific lesion locations in an image. We design the model to be parameter-efficient and thus resistant to over-fitting, which helps learning discriminative representations from large amount of data to effectively identify the lesions with a high classification accuracy. We use transfer learning with the pretrained AlexNet3 on ImageNet4 as the backbone following a max-pooling layer and a fully-connected layer for classification. This network is then fine-tuned on our BMLs training set. Details of our framework is seen in Figure 1. We evaluate our method using five widely-acknowledged metrics: (1) Accuracy, (2) Precision, (3) Recall, (4) F1 score, (5) Area under ROC curve (AUC).Results and discussion
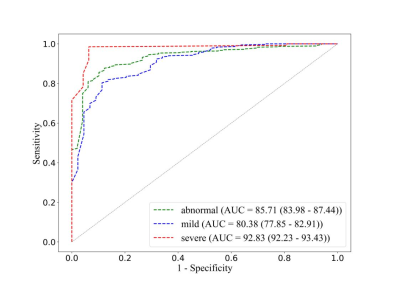
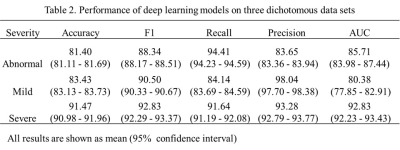
Our method yielded accuracy of 0.81 for distinguishing normal and abnormal bone marrow, and 0.84, 0.92 for separating normal bone marrow from mild BMLs and severe BMLs. Furthermore, our method yielded area under the ROC curve of 0.85, 0.80, 0.93 for detecting the presence of BMLs and varying severity of disease, respectively. The results are detailed in Table 2 and Figure 2. Although the dichotomous model shows great accuracy and high AUC, there exist limitations: the model only trained on 3D-DESS protocol, which is less common apply for clinical work. Therefore, the further step of our research is transferring the results to more sequences, improving its generalization.Conclusion
The deep learning-based dichotomous model performed can recognize the presence of BMLs accurately and initially assess of severity, making it meaningful to be a rapid detector of BMLs for associated research.Acknowledgements
Jiaping Hu and Zhao Wang contributed equally to this work.
Xiaodong Zhang and Qi Dou are both co-corresponding authors.
Funding: This project is supported by the National Natural Science Foundation of China (grant No. 81801653), and Guangdong Science and Technology Department (grant No. 2017B090912006) and start-up research grant from CUHK Research Committee.
References
1. Tanamas SK, Wluka AE, Pelletier JP, Pelletier JM, Abram F, Berry PA, Wang Y, Jones G. Bone marrow lesions in people with knee osteoarthritis predict progression of disease and joint replacement: a longitudinal study. Rheumatology (Oxford). 2010 Dec;49(12):2413-9
2. X. Jiny, B. Antonyy, X. Wang , et al. Radiographic and magnetic resonance imaging markers as predictors for total knee replacement over 10.7 years in older adults. 2016;24 (2016) :S63-S534
3. Krizhevsky A, Sutskever I, Hinton G E. Imagenet classification with deep convolutional neural networks[J]. Communications of the ACM, 2017, 60(6): 84-90.
4. Deng J, Dong W, Socher R, et al. Imagenet: A large-scale hierarchical image database[C]//2009 IEEE conference on computer vision and pattern recognition. Ieee, 2009: 248-255.
Figures