4054
DEMO: Deep MR Parametric Mapping using Unsupervised Multi-tasking Framework1Shenzhen Institutes of Advanced Technology, Chinese Academy of Sciences, Shenzhen, China
Synopsis
In this work, we propose a novel deep learning-based framework DEMO for fast and robust MR parametric mapping. Different from current deep learning-based methods, DEMO trains the network in an unsupervised way. Specifically, a CS-based loss function is used in DEMO to avoid the necessity of using fully sampled k-space data as the label, and thus make it an unsupervised learning approach. DEMO reconstructs the parametric weighted images and generates the parametric map simultaneously, which enables multi-tasking learning. Experimental results show the promising performance of the proposed DEMO framework in quantitative MR T1ρ mapping.
Introduction
Quantitative magnetic resonance (MR) parametric mapping is an emerging tool for evaluating and determining tissue's fundamental biologic properties. However, the relatively long reconstruction time restricts its widespread applications in the clinic1, 2. Compressed sensing (CS) has been investigated in MR parametric mapping to reduce the scan time3-6. Recently, deep learning-based methods have shown great potential in accelerating reconstruction time and improving imaging quality in fast MR imaging, whereas their adaptation to parametric mapping is still in an early stage7-9. The existing limited DL-based methods all use a deep network to reconstruct the parametric map from undersampled k-space data directly. Moreover, these methods conduct in a supervised manner where the reference parameter map is given. However, supervised learning needs a large number of fully sampled k-space data, which may be difficult in practice, and the reference parametric maps created by different fitting algorithms from fully sampled images may be slightly different. In this work, we propose a novel DEep MR parametric mapping method using unsupervised multi-tasking framewOrk, (DEMO), to handle the situation where collecting a large number of fully sampled k-space data of the parametric-weighted images is impractical. We use the mono-exponential T1ρ mapping of the knee as an example to demonstrate the feasibility of the proposed approach. The experimental results on in vivo data sets show that the proposed framework achieves superior reconstruction and mapping performance.Theory
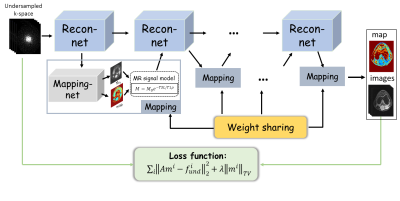
Given the fact that the parameter map is usually estimated from the parameter weighted images, DEMO adopts the CS objective function as loss function in network training. Specifically, the loss function used for network training in DEMO was defined as $$ L(\Theta)=\frac{1}{N}\sum_{j=1}^N||AM(\Theta,f^k)-f^k||_2^2+\lambda||M(\Theta,f^k)||_{TV} (1)$$ where $$$M(\Theta,f)$$$ is the parameter-weighted images based on network parameter $$$\Theta$$$ and undersampled k-space data $$$f$$$, $$$||.||_{TV}$$$ represents the total variation (TV) regularization. In such scenarios, no ground-truth maps or fully sampled data are needed.With deep networks, we proposed to simultaneously reconstruct the parameter-weighted images and the corresponding parametric map, where the whole procedure can be formulated as follows: $$ \begin{cases}m_{n+1}=\Gamma(m_n,\widetilde{m}_n,A^Hf)\\(M_0,T_x)_{n+1}= U(m_{n+1})\\\widetilde{m}=S(M_0,T_x)_{n+1}\end{cases} (1)$$ where $$$n$$$ is the iteration number, $$$m$$$ is the $$$T_x$$$-weighted images from deep reconstruction $$$\Gamma$$$, $$$(M_0,T_x)$$$ is the baseline image and associated $$$T_x$$$ map which are generated simultaneously from network $$$U$$$, $$$\widetilde{m}$$$ is the synthetic $$$T_x$$$-weighted images satisfying the $$$T_x$$$ signal decay.
Take T1ρ mapping for example, Fig 1 represents an overview of the proposed framework. There are two chained networks corresponding to the two tasks in Eq. (2): reconstruction task $$$\Gamma$$$ (Recon-net) and Mapping task $$$U$$$ (Mapping-net). The physical model $$$S$$$ is incorporated after Mapping-net to generate T1ρ-weighted images, which are then used as one of the inputs of the next Recon-net.
Method
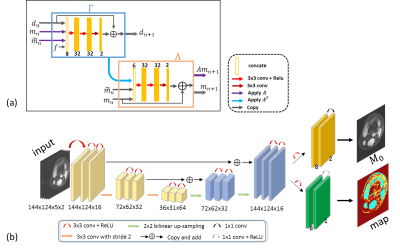
In this study, the PD-net architecture10 was modified for deep parameter-weighted image reconstruction, and a modified U-net architecture11 was adopted for generating a parametric map. The number of blocks was set to be 5, and the parameter was 0.0003. Five healthy volunteers were recruited for T1ρ scanning (4 used for training and one for testing), and informed consent was obtained from the imaging object in compliance with the IRB policy. All MR scans were performed on a 3T scanner (uMR 790, United Imaging Healthcare, Shanghai, China) using a commercial 12-channel phased-array knee coil. T1ρ-weighted images of the knee were acquired using a 3D MATRIX sequence and a self-compensated paired spin-lock preparation pulse. The imaging parameters were as follows: TE/TR = 8.96/2000 ms, matrix size: 256 × 144 × 124, TSLs = 5, 10, 20, 40, and 60 ms. The fully sampled multi-coil k-space data was adaptively combined to single-coil data, and then retrospectively undersampled using Poisson-disk masks with accelerations of 5.2.Results
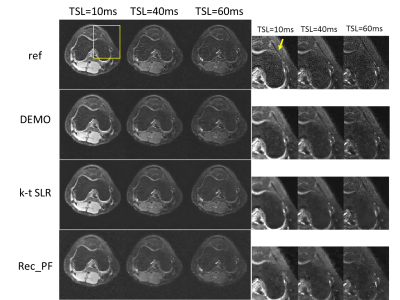
The performance of the proposed DEMO was evaluated by comparing with a sparsity driven method Rec_PF with the TV regularization12 and a low-rank and sparsity driven method k-t SLR13.Fig 3 shows the parameter-weighted image reconstructions from different reconstruction methods. Images reconstructed with rec-PF amd k-t SLR show apparent detail loss and noticeable artifacts. The proposed DEMO generates nearly artifact-free reconstructions with well-preserved features.
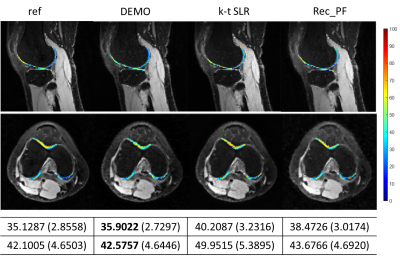
Fig 4 shows the overlaid reconstructions at R=5.2. The ROI T1ρ mean values and standard deviations of the different methods are also provided. DEMO gives the most similar values of T1ρ to the reference with the lowest standard deviation.
Conclusion
In this work, we proposed an efficient unsupervised DL-based framework for MR parametric mapping. Results on in vivo T1ρ knee imaging exhibit the superior performance of the proposed approach. The extension to other types of parametric mapping and more properties will be explored in the future.Acknowledgements
This work was supported in part by the National Key R&D Program of China (2017YFC0108802 and 2017YFC0112903); National Natural Science Foundation of China (61771463, 81830056, U1805261, 81971611, 61871373, 81729003, 81901736); Natural Science Foundation of Guangdong Province (2018A0303130132); Key Laboratory for Magnetic Resonance and Multimodality Imaging of Guangdong Province; Shenzhen Peacock Plan Team Program (KQTD20180413181834876); Innovation and Technology Commission of the government of Hong Kong SAR (MRP/001/18X); Strategic Priority Research Program of Chinese Academy of Sciences (XDB25000000).References
[1] Duvvuri U, Charagundla S, Kudchodkar S, et al. Human knee: in vivo T1(rho)-weighted MR imaging at 1.5 T—preliminary experience. Radiology 2001, 220(3):822-6.
[2] Regatte R, Akella S, Lonner J, et al. T1rho relaxation mapping in human osteoarthritis (OA) cartilage: comparison of T1rho with T2. J Magn Reson Imaging 2006, 23(4):547-53.
[3] Petzschner F, Ponce I, Blaimer M, et al. Fast MR parameter mapping using k-t principal component analysis. Magn Reson Med 2011, 66(3):706-716.
[4] Velikina J, Alexander A, and Samsonov A. Accelerating MR Parameter Mapping Using Sparsity-Promoting Regularization in Parametric Dimension. Magn Reson Med 2013, 70(5): 1263-1273.
[5] Zhou Y, Pandit P, Pedoia V, et al. Accelerating T1rho cartilage imaging using compressed sensing with iterative locally adapted support detection and JSENSE. Magn Reson Med 2016, 75(4):1617-1629. [6] Pandit P, Rivoire J, King K, et al. Accelerated T1rho acquisition for knee cartilage quantification using compressed sensing and data-driven parallel imaging: A feasibility study. Magn Reson Med 2016, 75(3):1256-1261.
[7] Cai C, Wang C, Zeng Y, et al. Single-shot T2 mapping using overlapping-echo detachment planar imaging and a deep convolutional neural network. Magn Reson Med 2018, 80(5):2202-2214.
[8] Liu F, Feng L, and Kijowski R. MANTIS: Model-Augmented Neural neTwork with Incoherent k-space Sampling for efficient MR parameter mapping. Magn Reson Med 2019, 82(1):174-188.
[9] Cohen O, Zhu B, and Rosen M. MR fingerprinting Deep RecOnstruction NEtwork (DRONE). Magn Reson Med 2018, 8(3):885-894.
[10] Cheng J, Wang H, Ying L, Liang D. Model Learning: Primal Dual Networks for Fast MR Imaging. MICCAI 2019
[11] Ronneberger O, Fischer P, Brox T. U-Net: convolutional networks for biomedical image segmentation. MICCAI 2015
[12] Yang J, Zhang Y, and Yin W. A Fast Alternating Direction Method for TVL1-L2 Signal Reconstruction From Partial Fourier Data. IEEE J Sel Top Signal Process 2010, 4(2):288-297.
[13] Lingala S, Hu Y, DiBella E, et al. Accelerated dynamic MRI exploiting sparsity and low-rank structure: k-t SLR. IEEE Trans Med Imaging 2011, 30(5):1042-54.
Figures