4052
Deep-Learning Based Image Reconstruction for Lumbar Spine MRI at 3T: Clinical Feasibility
Emma Bahroos1, Misung Han1, Cynthia Chin1, David Shin2, Javier Villanueva-Meyer1, Thomas Link1, Valentina Pedoia1, and Sharmila Majumdar1
1Radiology and Biomedical Imaging, University of California San Francisco, San Francisco, CA, United States, 2Applications and Workflow, GE Healthcare, Menlo Park, CA, United States
1Radiology and Biomedical Imaging, University of California San Francisco, San Francisco, CA, United States, 2Applications and Workflow, GE Healthcare, Menlo Park, CA, United States
Synopsis
Lower back pain is one of the most common health problems, for which MRI is extensively used. Standard clinical, and fast acquisition images of lumbar spine were acquired for 18 patients. A (DL)-based image reconstruction was applied to the raw data of the fast images, with 25%, 50%, and 75% noise reduction factors. Evaluation of fast images with DL algorithm, for image quality, diagnostic capability, and SNR to standard images was conducted by three experienced radiologists. Our results show SNR improvement with higher noise reduction factor without a severe degradation in the ability to discern anatomical structures.
Introduction
Lower back pain (LBP) is one of the most common health problems, responsible for limiting ones daily activities and work, and ultimately a cause of deteriorating quality of life1. Magnetic resonance imaging (MRI) is extensively used in clinical diagnosis of LBP, however long scan times have always been a challenge and a hindrance to patient comfort. Previous studies have shown accelerated MRI can be performed with deep learning (DL) algorithm for image reconstruction2,3 in the knee and brain4,5, however not many studies have been done on lumbar spine. The aim of this study was to evaluate whether fast image acquisition, combined with a DL reconstruction algorithm were equivalent to standard clinical acquisitions concerning image quality, diagnostic capability, and image analysis.Methods
Image acquisition and reconstructionOur institutional lumbar spine MRI protocol consists of 2D fast spine echo T1 and T2 sequences in multiple planes (Table 1). These clinical sequences use a signal averaging (NEX) of 1-2.5 to achieve SNR for a high diagnostic confidence. To this routine clinical protocol, we added a fast acquisition protocol (shown in Table 1) with NEX reduced to 0.5-1. Fast acquisition along with the routine clinical sequences in 18 patients were acquired on a GE 3T SIGNA Premier scanner (GE Healthcare, Waukesha, WI) with an embedded GEM coil array.
A deep-learning (DL)-based image reconstruction was applied to the raw k-space data of the reduced NEX fast acquisition protocol, with three different noise reduction factors, 25%, 50%, and 75%. This DL-recon algorithm was constructed using a deep convolutional residual encoder network trained with a database of more than a 10,000 images, designed to improve SNR and while maintaining sharpness of MR images6.
Image analysis and grading
The sagittal fat-saturated T2, sagittal T1, and axial T2 weighted images from the standard clinical as well as fast acquisitions, and DL-reconstructed images from the fast acquisitions (a total of 15 series per patient) from 18 patients were anonymized. All image details were removed and all image series were randomly ordered for each sequence method, and presented to three experienced radiologists to grade based on 1) apparent signal-to-noise ratio (SNR); 2) ability to discern anatomical structures; 3) diagnostic confidence; 4) overall image quality; and 5) artifacts. The first 4 metrics were rated on a Likert scale of 1 to 5 (5=excellent) while the last metric was rated as binary (1=artifact(s) present, 2=no artifacts).
Wilcoxon sign-rank test was performed to assess significant differences between standard and the fast acquisition with DL-reconstruction images. A p-value lower than 0.05, suggested there was a difference (the median change was not zero)and the direction of the change was determined (better or worse than the standard images).
Results
The fast images provided lower SNR than the standard images as expected, but DL reconstruction reduced image noise effectively. SNR improvement over higher noise reduction factor was found without degrading the ability to discern anatomical structures. Figure 1 shows comparison images of a patient with L2 to L4 laminectomy post-surgery. Fluid along the surgical tract and Modic type II changes in multiple vertebral bone marrow and facet hypertrophy can be seen on all images with similar quality. In another example, (Figure 2) a large disc extrusion is seen on images of a patient with history LBP for 6 weeks, lower extremity weakness and lumbar radiculopathy.The average ratings from three radiologists of the four criteria (‘artifacts’ is not included in this figure) of 18 patient data sets is summarized in Figure 3, as box plots. The Fast DL50 and DL75 images appear to be providing comparable results to that of the standard imaging protocol for sagittally acquired images. Table 2 summarizes the p-values of each dataset, when compared to standard images. Overall the DL-reconstructed sagittal images (DL 50 & DL75, p>0.05) did not show a significant difference from the routine clinical protocol. For axial plane, DL 25 and DL 50 (p>0.05) seemed to perform similar to the standard images.
Discussion & Conclusion
This study demonstrated that the DL reconstruction reduced image noise effectively, providing a higher SNR with a higher noise reduction factor, without degrading depiction of anatomical structures, especially for images in the sagittal plane. Scan times can be cut in half using a reduced NEX protocol and loss of SNR can be recovered by a DL algorithm. Ultimately, this tool provides a potential for faster lumbar spine imaging, and subsequently a path for an efficient clinical workflow for patients with severe LBP.Acknowledgements
This project was supported by the BACPAC grant UH2AR076724 (SM).References
- D. Hoy, L. March, P. Brooks, A. Woolf, F. Blyth, T. Vos, and R. Buchbinder, "Measuring the global burden of low back pain," Best Pract Res Clin Rheumatol 24 (2), 155-165 (2010).
- M. P. Recht, J. Zbontar, D. K. Sodickson, F. Knoll, N. Yakubova, A. Sriram, T. Murrell, A. Defazio, M. Rabbat, L. Rybak, M. Kline, G. Ciavarra, E. F. Alaia, M. Samim, W. R. Walter, D. J. Lin, Y. W. Lui, M. Muckley, Z. Huang, P. Johnson, R. Stern, and C. L. Zitnick, "Using Deep Learning to Accelerate Knee MRI at 3 T: Results of an Interchangeability Study," AJR Am J Roentgenol 215 (6), 1421-1429 (2020).
- M. Kidoh, K. Shinoda, M. Kitajima, K. Isogawa, M. Nambu, H. Uetani, K. Morita, T. Nakaura, M. Tateishi, Y. Yamashita, and Y. Yamashita, "Deep Learning Based Noise Reduction for Brain MR Imaging: Tests on Phantoms and Healthy Volunteers," Magn Reson Med Sci 19 (3), 195-206 (2020).
- K. Hammernik, T. Klatzer, E. Kobler, M. P. Recht, D. K. Sodickson, T. Pock, and F. Knoll, "Learning a variational network for reconstruction of accelerated MRI data," Magn Reson Med 79 (6), 3055-3071 (2018).
- B. Zhu, J. Z. Liu, S. F. Cauley, B. R. Rosen, and M. S. Rosen, "Image reconstruction by domain-transform manifold learning," Nature 555 (7697), 487-492 (2018).
- R. Marc Lebel, "Performance characterization of a novel deep learning-based MR image reconstruction pipeline", (2020), pp. arXiv:2008.06559.
Figures

Table 1: Lumbar Spine MRI UCSF Standard Clinical and Fast Protocols

Figure 1: Comparing images from the standard and fast acquisitions and DL-reconstructed images (Standard, Fast, Fast DL25, Fast DL50, Fast DL75 images). The mean scores from the three radiologists for the ‘overall image quality’ is stated on each image. Changes in multiple vertebral bone marrow can be seen on sagittal images (depicted by solid and dashed arrows, respectively), and facet hypertrophy (depicted by an arrow) on axial images.

Figure 2: Comparing images from the standard and fast acquisitions and DL-reconstructed images (Standard, Fast, Fast DL25, Fast DL50, Fast DL75 images). The mean scores from the three radiologists for the ‘overall image quality’ is stated on each image. Large disc protrusion is depicted with the arrow on each sequence.

Figure 3: The scores for Standard,
Fast, Fast DL25, Fast DL50, and Fast DL75 images were separately drawn for each
sequence. DL-reconstructed images show an improved SNR and the ability to
discern anatomical structures, and equivalent diagnostic capability, for all of
the sagittal fat-saturated T2-weighted, sagittal T1-weighted
images, and axial T2-weighted sequences. The overall image quality
for DL-reconstructed Sag T1 and Sag T2 FS were comparable to the standard
routine images.
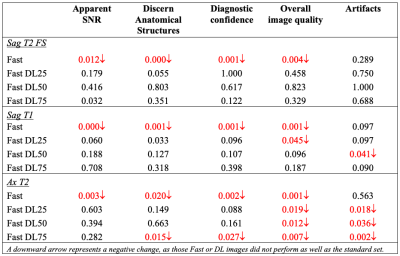
Table 2: Statistical analysis: P-values of the Wilcoxon sign-rank
test (p>0.05, for no significant difference) comparing standard images to
the fast and DL-reconstructed images.