4049
Differentiation of Benign and Malignant Vertebral Fractures on Spine MRI Using ResNet Deep Learning Compared to Radiologists’ Reading1Radiology, E-Da Hospital, Kaohsiung, Taiwan, 2University of California Irvine, Irvine, CA, United States, 3Radiology, Chi-Mei Medical Center, Tainan, Taiwan
Synopsis
This study compared
the reading of three radiologists with different level of experience, and also investigated
the potential of deep learning to differentiate between benign and malignant
vertebral fractures based on T1W and T2W MRI. The results showed that deep
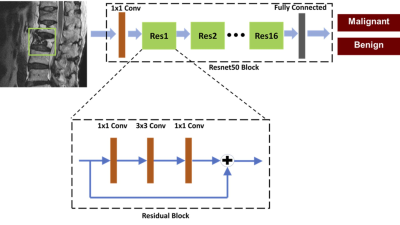
learning using ResNet50 achieved a satisfactory diagnostic accuracy of 92%,
although inferior to 98% made by a senior MSK radiologist and 96% made by a R4
resident, much higher compared to 66% made by a R1 resident. The inferior
performance of ResNet50 might be partly explained by the very limited
information when only considering a small bounding box.
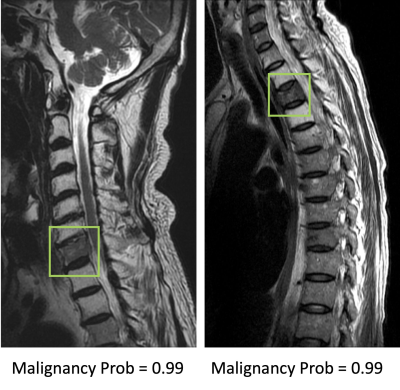
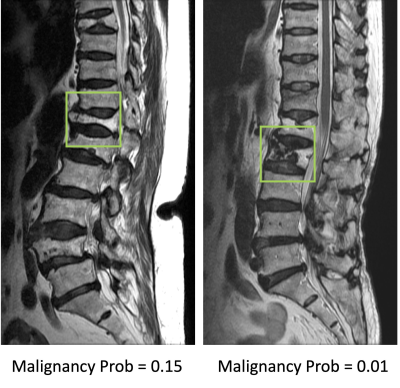
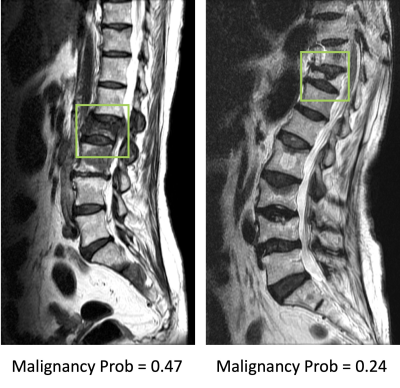
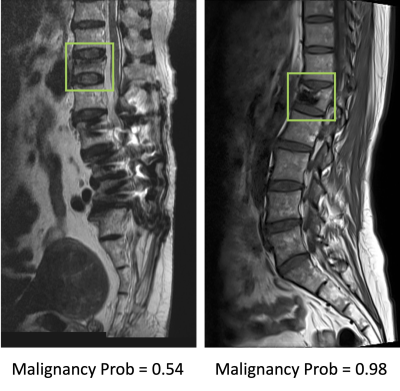
Results: The senior MSK radiologist’s accuracy was 0.98. The 4th year resident also had very high accuracy of 0.96, and not surprisingly, the first year resident performed poorly with accuracy of 0.66. When individual scores of 15 features were used to build a logistic regression model, the diagnostic accuracy was 0.94. Diffuse signal changes occurred more frequently in the malignant group (88%). Intravertebral dark lines or bands were present only in benign fractures (26%). When deep learning using ResNet50 was applied, the accuracy was 0.84 for per-slice diagnosis, and 0.92 for per-patient diagnosis. There were 3 false negative and 12 false positive diagnoses. Figure 2 shows two malignant cases correctly diagnosed as true positives. Figure 3 shows two benign cases correctly diagnosed as true negatives. Figure 4 shows two malignant cases misdiagnosed as benign, and Figure 5 shows two benign cases misdiagnosed as malignant. These mis-diagnosed cases by deep learning were all correctly diagnosed by the senior MSK radiologist, and the important features are described in the figure legends.
Conclusions: This study investigated the application of deep learning for the differential diagnosis of benign and malignant vertebral fracture on MRI. These results suggest that deep learning using ResNet50 provides a feasible method to use T1-weighted and T2-weighted images on MRI to establish a diagnosis. The input used in deep learning was a square box covering a single abnormal vertebral body, without the inclusion of soft tissue, posterior elements, and skipped lesions. The per-patient diagnostic accuracy was 0.92, which was inferior to reading of radiologists who had sufficient training, but much higher than that of an inexperienced radiologist. The results suggest that the developed ResNet50 model may have a good clinical value in facilities lack of well-trained medical staff. With specific refinement in each clinical setting, this AI-based method has the potential to serve as a clinical tool to help less experienced readers and to improve workflow.
Acknowledgements
This study was supported by E-Da Hospital intramural seed grant EDAHM108003, NIH R01 CA127927.References
1. Avellino AM, Mann FA, Grady MS, et al. The misdiagnosis of acute cervical spine injuries and fractures in infants and children: the 12-year experience of a level I pediatric and adult trauma center. Child's Nervous System. 2005;21:122-127.
2. Diacinti D, Vitali C, Gussoni G, et al. Misdiagnosis of vertebral fractures on local radiographic readings of the multicentre POINT (Prevalence of Osteoporosis in INTernal medicine) study. Bone. 2017;101:230-5.
3. Bengio Y. Learning deep architectures for AI. Foundations and trends® in Machine Learning. 2009;2:1-127.
Figures