4048
Assessment of the potential of a Deep Learning Knee Segmentation and Anomaly Detection Tool in the clinical routine
Laura Carretero1, Pablo García-Polo1, Suryanarayanan Kaushik 2, Maggie Fung2, Bruno Astuto3,4, Rutwik Shah3,4, Pablo F Damasceno3,4, Valentina Pedoia3,4, Sharmila Majumdar3,4, and Mario Padrón5
1Global Research Organization, GE Healthcare, Madrid, Spain, 2GE Healthcare, Waukesha, WI, United States, 3Department of Radiology and Biomedical Imaging, UCSF, San Francisco, CA, United States, 4Center for Digital Health Innovation, UCSF, San Francisco, CA, United States, 5Department of Radiology, Clínica Cemtro, Madrid, Spain
1Global Research Organization, GE Healthcare, Madrid, Spain, 2GE Healthcare, Waukesha, WI, United States, 3Department of Radiology and Biomedical Imaging, UCSF, San Francisco, CA, United States, 4Center for Digital Health Innovation, UCSF, San Francisco, CA, United States, 5Department of Radiology, Clínica Cemtro, Madrid, Spain
Synopsis
This study evaluates the clinical accuracy of a deep learning (DL)-based tool to segment articular cartilage and menisci on 50 knee MRI exams; detect lesions and stage its severity. An experienced MSK radiologist assessed independently the images for the presence of any lesions on the different compartments and checked the accuracy of its segmentation, resulting in no disagreement with the segmentation output in 92.8% of the compartments and correspondence in the detection of lesions in 75.94% of them. The shown results assessed the clinical potential of this tool and present a step forward into structured MSK imaging reports.
Background
Recent developments of DL-based algorithms have been focused on automatic segmentation and bone and soft tissue abnormalities detection in musculoskeletal (MSK) imaging1,2. Automatic segmentation and morphological grading of joint tissue can have a significant impact in osteoarthritis (OA) research and clinical settings. The inclusion of quantitative biomarkers will improve patient outcomes by saving reporting time per exam and help generating robust radiological reports3,4.The main purpose of this study was to assess the potential of a new DL-based MR knee cartilage segmentation algorithm5-7 and its impact in both patients’ workflow and OA clinical research. The tool automatically segments articular cartilage and menisci; grades the health of the cartilage and computes the lesion probability in the different compartments.
According to the tool performance, the following areas were evaluated:
- Tissue segmentation accuracy
- Lesion detection and classification accuracy
- Overall clinical utility
Subject Population
50 patients with a scheduled knee exam, prior consent form signature, were recruited in Clinica Cemtro, Madrid (Spain) for this study.Data Acquisition Methods
All MRI examinations were conducted on a GE Healthcare (Waukesha, WI) 3T SIGNA™ Architect scanner with an 18-Ch Tx/Rx extremity coil, with the following addition to the routine knee clinical protocol: Fat-suppressed 3D FSE CUBE sequence, repetition time (TR)=1200ms, minimum echo time (TE)=28ms, field of view (FOV)=16 cm, acquisition matrix =260×260, slice thickness=0.6mm, echo train length = 40, bandwidth =50.0kHz, number of excitations (NEX)=1, HyperSense (compressed sensing) factor=1.2, ARC acceleration 2x2, sagittal view, acquisition time=5 minutes.This sequence was processed using the segmentation tool, which provided an additional DICOM series with the segmentation output, a CSV file with comprehensive anomaly detection and lesion probability results, and a visual report with lesion probabilities in each compartment (Figure 1).
Data Analysis Methods
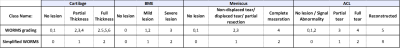
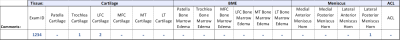
To evaluate the lesion classification accuracy, an MSK expert radiologist (25 years’ experience) independently assessed the images for the presence of any lesions on the bone marrow, cartilage, menisci or anterior cruciate ligament (ACL).This classification system was developed from the Whole Organ Magnetic Resonance Imaging Score (WORMS)8, by simplifying the subregions to focus on the key degenerative features and grouped the lesion grading into three grades (Figure 2) – 6 subregions for cartilage and bone marrow edema (BME): Patella, Trochlea, Medial and Lateral Femur (LF, MF), Medial and Lateral Tibia (MT, LT); and 4 regions for Meniscus: Medial anterior and posterior horn, Lateral anterior and posterior horn (Figure 3).
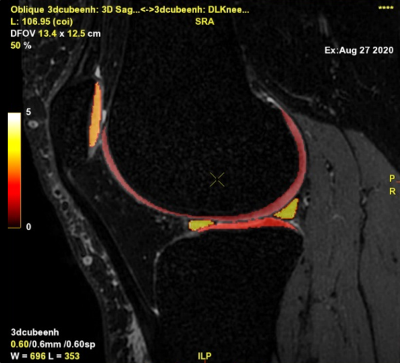
To evaluate the segmentation performance, the segmentation output series was fused over the CUBE sequence for each case (Figure 4). Its accuracy was visually assessed in each compartment using a 4-point Likert scale in case of disagreement between the radiologist and the DL tool (1 - Slight (< 5%), 2 - Moderate (5% to 20%), 3 - Substantial (20% to 50%), 4 - Strong Disagreement (>50%)). The thickness of each segmentation or any other issue was also captured, and the affected slices were specified when possible (Figure 5).
Results
The study shows a good match between the segmentation outputs and the radiologist’s criteria. We grouped the level of disagreement in the five tissue compartments resulting in no disagreement in 92.8% of the compartments, slight in 0.8%, moderate in 1.6%, substantial in 0.4% and strong disagreement in 4.4% of them. Some of the errors were due to the lack of femoral cartilage near the LCA in a few slices and some extra tissue segmentation of subchondral cysts, fluid and fat outside the limits of the regions of interest.Regarding anomaly detection accuracy, the algorithm outcome and the expert’s criteria agreed in the presence of lesion in 75.94% of the tissue compartments. In 11.44% of the compartments the tool does not detect the anomaly identified by the expert, whereas in 12.62% it extracts secondary small lesions which were unnoticed by the reader. From the underestimated cases, almost 50% corresponds to BME misclassifications – the diagnosis was more severe with visual assessment.
Discussion
Issues encountered in segmentation were not key to diagnosing. Regarding lesion detection, patellar and trochlear cartilage weren’t well graded in a few cases, likely since the tool was trained in the sagittal plane, whereas the radiologist has the 3D reformatted visualization. While the tool lacked the accuracy for detecting and grading BME, it was able to detect slight lesions which is potentially useful for early diagnosis.From a clinical utility standpoint, this tool would facilitate the generation of structured reports – improving and speeding up radiologists’ daily routines, who would check those regions highlighted with some degree of lesion probability.
In addition, the segmentation could potentially be fused with other functional sequences for cartilage assessment, having a better and accurate understanding of cartilage structure, composition and behavior.
Conclusion
The ability of detecting lesions automatically while the subject is still in the scanner could allow the MRI protocol to be adjusted to the patient needs, which is a good approach to precision medicine. DL-generated reports with lesion probability implies a great step into structured MSK imaging reports. Furthermore, the addition of lesion severity color-coded maps and the possibility of building 3D models with the segmented tissues, adds great value to the overall clinical workflow by involving the patient in their own diagnoses and treatment.Acknowledgements
No acknowledgement found.References
- Pedoia V, Majumdar S, Link T. Segmentation of joint and musculoskeletal tissue in the study of arthritis. Magnetic Resonance Materials in Physics, Biology and Medicine. 2016;29(2):207-221.
- Bach Cuadra M, Favre J, Omoumi P. Quantification in Musculoskeletal Imaging Using Computational Analysis and Machine Learning: Segmentation and Radiomics. Seminars in Musculoskeletal Radiology. 2020;24(01):50-64.
- Pedoia V, Majumdar S. Translation of morphological and functional musculoskeletal imaging. Journal of Orthopaedic Research. 2019;37(1):23-34.
- Menashe L, Hirko K, Losina E, et al. The diagnostic performance of MRI in osteoarthritis: a systematic review and meta-analysis. Osteoarthritis Cartilage. 2012;20(1):13–21
- Pedoia V, Norman B, Mehany SN et al. 3D Convolutional Neural Networks for Detection and Severity Staging of Meniscus and PFJ Cartilage Morphological Degenerative Changes in Osteoarthritis and Anterior Cruciate Ligament Subjects. J Magn Reson Imaging 2019;49(2):400–10.
- Astuto B, Namiri NK, Flament I, et al. Deep Learning Assisted Full Knee 3D MRI-Based Lesion Severity Staging. In: ISMRM 28th Annual Meeting & Exhibition (Virtual) August. 2020.
- Astuto B, Flament I, Namiri NK, et al. Automatic Deep Learning Assisted Detection and Grading of Abnormalities in Knee MRI Studies. Radiology Artificial Intelligence. (Under Revision)
- Peterfy CG, Guermazi A, Zaim S, et al. Whole-Organ Magnetic Resonance Imaging Score (WORMS) of the knee in osteoarthritis. Osteoarthritis Cartilage, 2004;12(3):177-90.