4045
Characterizing Knee Osteoarthritis Progression with Structural Phenotypes using MRI and Deep Learning1Department of Radiology and Biomedical Imaging and Center for Intelligent Imaging, University of California, San Francisco, San Francisco, CA, United States
Synopsis
We built an end-to-end deep learning model to rapidly stratify knees into morphological phenotypes using a large, longitudinal cohort with knee osteoarthritis (OA). We examined associations of phenotypes with odds of concurrent OA and OA progression. Bone, meniscus/cartilage, and inflammatory phenotypes were strongly associated with current structural OA and symptomatic OA. Hypertrophy phenotype was only weakly associated with structural OA. Among those who did not have baseline OA, bone and meniscus/cartilage phenotypes were strongly associated with developing both structural and symptomatic OA in 48 months. Only bone phenotype increased risk of undergoing total knee replacement surgery within 96 months.
Introduction
Osteoarthritis (OA) develops through heterogeneous pathophysiologic pathways, a primary reason there are not yet regulatory agency approved disease modifying OA drugs to date.1–3 Rapid OsteoArthritis MRI Eligibility Score (ROAMES) was recently introduced as a simplified MRI assessment metric for stratification of knees into morphological phenotypes potentially applicable to determine eligibility for disease-modifying OA drug trials.4 A small pilot study demonstrated a potential correlation between these phenotypes and OA progression.5 Although promising, larger cohort studies with MRI assessment are needed to affirm the prognostic value of morphological phenotypes in predicting future OA incidence. Our aim was to build a fully automatic end-to-end deep learning model to stratify knees into ROAMES phenotypes (i.e. bone, meniscus/cartilage, inflammatory, hypertrophy) and evaluate the prevalence and association of phenotypes with structural and symptomatic knee OA.Methods
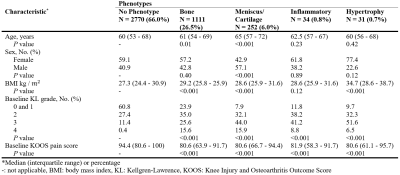
We collected coronal intermediate-weighted two-dimensional turbo spin-echo and two-dimensional sagittal intermediate-weighted fat-suppressed turbo spin-echo knee MRIs obtained using 3T scanners from participants (n=4,791) in the OsteoArthritis Initiative (OAI) at all clinic visits.6–8 A subset of images (n=4,413) were graded by a centralized group under supervision of two musculoskeletal radiologists with more than nine years of training in semi-quantitative knee OA grading.8 The sample size of cases and controls for bone, meniscus/cartilage, inflammatory, and hypertrophy were 532 and 3109, 101 and 3535, 50 and 1906, and 57 and 543, respectively. The radiologist-graded images were split into training (70%), validation (10%), and test sets (20%) to train a convolutional neural network (CNN) for each ROAMES phenotype, preserving the distributions of baseline demographics, radiographic severity, and pain severity. The CNNs used MRNet neural network architecture, which utilize each slice of the concatenated coronal and sagittal views as input into an ImageNet pre-trained AlexNet for feature extraction.9 The CNNs were then utilized to predict phenotypes for the entire cohort’s bilateral knee images (n=45,300 knees). We investigated the association between (1) baseline phenotypes and current structural OA (Kellgren-Lawrence (KL)10 radiographic grading scheme greater than 2) and symptomatic OA (presence of pain, aching, or stiffness in knee joint in past 12 months) among all participants using logistic regression, (2) baseline phenotypes and incidence of OA in 48 months among participants without OA at baseline using mixed effects logistic regression analyses to account for multiple observation by participants, and (3) phenotypes and undergoing a primary total knee replacement (TKR) after baseline and prior to the 96-month visit using logistic regression. All models were adjusted for baseline characteristics including age, sex, race, and body mass index (BMI).Results
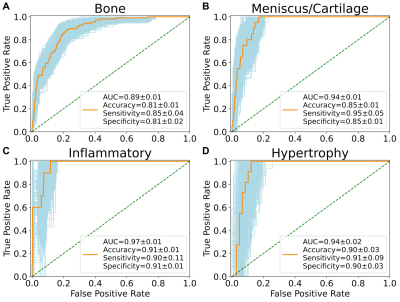
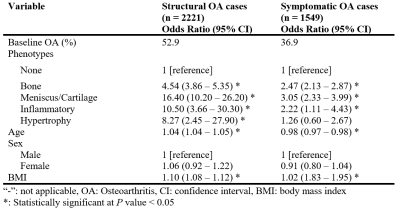
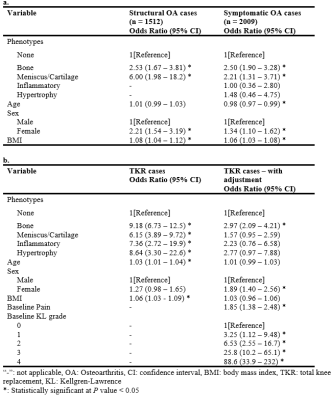
There were no statistically significant differences between participants in the training, validation, and test sets regarding demographics, radiographic scores, and pain scores (Table 1). The area under curve (AUC) of bone, meniscus/cartilage, inflammatory, and hypertrophy CNN classifiers were 0.89±0.01, 0.94±0.01, 0.97±0.01, and 0.94±0.02, respectively (Figure 1). The final cohort included 4,198 unique knees. At baseline, the cohort contained 1,111 (26.5%) bone phenotype, 252 (6.0%) meniscus/cartilage phenotype, 34 (0.8%) inflammatory phenotype, 31 (0.7%) hypertrophy phenotype, and 2,770 (66%) no phenotype (Table 2). Participants at baseline in bone (OR:4.54; 95%CI:3.86–5.35), meniscus/cartilage (OR:16.40; 95%CI:10.20–26.20), inflammatory (OR:10.50; 95%CI:3.66–30.30), and hypertrophy (OR:8.27; 95%CI:2.45–27.90) phenotype groups had significantly more structural OA than those in no phenotype group (Table 3). Symptomatic OA was significantly higher among participants in bone (OR:2.47; 95%CI:2.13–2.87), meniscus/cartilage (OR:3.05; 95%CI:2.33–3.99), and inflammatory (OR:2.22; 95%CI:1.11–4.43) phenotype groups than those in no phenotype group. Participants in both bone (OR:2.53; 95%CI:1.67–3.81) and meniscus/cartilage phenotypes (OR:6.00; 95%CI:1.98–18.2) had significantly higher adjusted odds of developing structural OA in 48 months compared to no phenotype (Table 4a). Among those without symptomatic OA at baseline, bone (OR:2.50; 95%CI:1.90–3.28) and meniscus/cartilage (OR:2.21; 95%CI:1.31–3.71) phenotypes possessed significantly higher odds of developing symptomatic OA in 48 months. All four phenotypes were associated with significantly increased odds of undergoing TKR within 96 months (Table 4b). After adjustment for baseline KL grades and presence of pain, aching, and stiffness in knee joint at baseline, bone remained the only phenotype to significantly increase odds of undergoing TKR within 96 months (OR:2.97; 95%CI:2.09–4.21).Discussion
Roemer et al. conducted a study associating ROAMES phenotypes with OA in a cohort of 485 knee MRIs.5 They reported knees with bone phenotype at baseline had highest odds of structural OA at either 24, 36, or 48 months (OR:1.87; 95%CI:1.18–2.97). However, neither bone, meniscus/cartilage, nor inflammatory phenotypes increased odds of pain progression over the same study period. Our study found bone phenotype increased odds of structural OA and symptomatic OA both at baseline and at 48 months. The difference in findings between Roemer et al. and our study may be because we did not exclude knees based on KL grade, whereas Roemer et al. excluded all knees with KL less than 2. Both studies also had different sample sizes and study lengths.Conclusion
Deep learning can rapidly stratify knees into structural phenotypes in a large, longitudinal cohort with knee OA. The study underscores the prognostic value of phenotypes for characterizing knee OA progression. These findings hold implications for improving understanding of OA pathogenesis, which may guide inclusion criteria of disease-modifying OA drug trials towards MRI-based structural phenotypes.Acknowledgements
No acknowledgement found.References
1. Karsdal MA, Michaelis M, Ladel C, et al. Disease-modifying treatments for osteoarthritis (DMOADs) of the knee and hip: lessons learned from failures and opportunities for the future. Osteoarthr Cartil 2016;24(12):2013–21.
2. Oo WM, Yu SP-C, Daniel MS, Hunter DJ. Disease-modifying drugs in osteoarthritis: current understanding and future therapeutics. Expert Opin Emerg Drugs 2018;23(4):331–47.
3. Van Spil WE, Kubassova O, Boesen M, Bay-Jensen A-C, Mobasheri A. Osteoarthritis phenotypes and novel therapeutic targets. Biochem Pharmacol 2019;165:41–8.
4. Roemer FW, Collins J, Kwoh CK, et al. MRI-based screening for structural definition of eligibility in clinical DMOAD trials: Rapid OsteoArthritis MRI Eligibility Score (ROAMES). Osteoarthr Cartil 2020;28(1):71–81.
5. Roemer FW, Collins JE, Neogi T, Crema MD, Guermazi A. Association of knee OA structural phenotypes to risk for progression: a secondary analysis from the Foundation for National Institutes of Health Osteoarthritis Biomarkers study (FNIH). Osteoarthr Cartil 2020;
6. Felson DT, Niu J, Gross KD, et al. Valgus malalignment is a risk factor for lateral knee osteoarthritis incidence and progression: findings from the Multicenter Osteoarthritis Study and the Osteoarthritis Initiative. Arthritis Rheum 2013;65(2):355–62.
7. Badlani JT, Borrero C, Golla S, Harner CD, Irrgang JJ. The effects of meniscus injury on the development of knee osteoarthritis: data from the osteoarthritis initiative. Am J Sports Med 2013;41(6):1238–44.
8. Roemer FW, Kwoh CK, Hannon MJ, et al. Can structural joint damage measured with MR imaging be used to predict knee replacement in the following year? Radiology 2015;274(3):810–20.
9. Bien N, Rajpurkar P, Ball RL, et al. Deep-learning-assisted diagnosis for knee magnetic resonance imaging: development and retrospective validation of MRNet. PLoS Med 2018;15(11):e1002699.
10. Kohn MD, Sassoon AA, Fernando ND. Classifications in brief: Kellgren-Lawrence classification of osteoarthritis. 2016;
Figures