4035
A Linear Gradient Solenoid for Slice-Selective Brain Imaging using B1+-Selective RF Pulses1Biomedical Engineering, Vanderbilt University, Nashville, TN, United States, 2Institute of Imaging Science, Vanderbilt University, Nashville, TN, United States, 3Radiology, Vanderbilt University, Nashville, TN, United States, 4Electrical Engineering, Vanderbilt University, Nashville, TN, United States
Synopsis
Despite drawbacks including production of acoustic noise and peripheral nerve simulation, long switching times, bulkiness, and high costs, use of B0 gradient coils is ubiquitous in conventional MR imaging. Replacement of B0 encoding with B1+ encoding alleviates these concerns and allows the use of hardware which is lower-cost, more compact, and silent. As a first step towards realizing a full B1+ encoding coil system, this work demonstrates an RF z-gradient solenoid coil developed for brain imaging on a 47.5 mT system. The coil performance is evaluated in simulation, bench, and scanner experiments and slice-selection using B1+-selective pulses is demonstrated.
Introduction
In conventional MRI, acquired signals are localized using B0 gradients which link spatial position to signal frequency and/or phase. However, the coils used to produce these gradient fields suffer numerous drawbacks including acoustic noise production, peripheral nerve stimulation, long switching times, bulk, and cost. Replacement of B0 encoding with B1+ encoding was suggested early in the history of MRI[1] and alleviates these concerns but has historically encountered difficulties in translating existing sequences to those using B1+ encoding while maintaining orthogonality between spatial encoding and image contrast.Recent developments on pre-clinical systems[2-4], including advances in B1+-selective excitation pulse design[4] have alleviated many these concerns. However, many of these advances have yet to be translated to in vivo human imaging, and RF coils producing the appropriate B1+ gradients must be developed to employ these methods. As a first step towards demonstration of full encoding, this work presents a coil system capable of performing in vivo slice-selection on a 47.5 mT system, a field strength especially attractive considering recent interest in improving scanner accessibility.
Methods
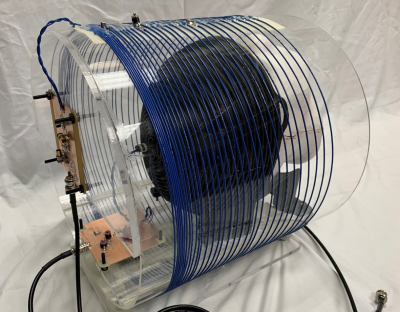
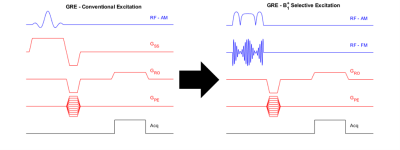
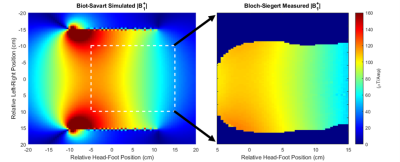
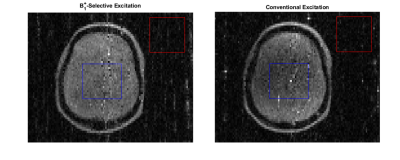
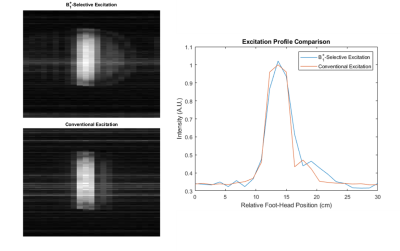
A 32-turn, variable-pitch solenoid (diameter: 30 cm, length: 20 cm) shown in Figure 1 was designed for use with a 47.5 mT permanent magnet system to produce a B1+ amplitude z-gradient over the brain volume. A quadratically-increasing pitch was chosen based on Biot-Savart modeling indicating this design would produce a linearly decreasing field with suitable strength and linearity across a 15 cm length (B1 +max = 105 μT/A, dB1+/dz = -0.042 G/cm/A, R2 = 0.99). The coil was wound around an acrylic former with 22 AWG wire and three capacitive breaks, one of which included a trimmer for fine-tuning the coil to the 2.07 MHz magnet frequency. A capacitive L-network was used for matching. Though the presented data used the coil in transmit/receive mode, the coil incorporates fast-switching crossed-diodes within its windings for passive detuning so it can accommodate a separate, detunable head solenoid for signal reception based on a modified spiral design[5,6].For all scanner experiments, the coil was fully enclosed in a copper shielding box for EMI reduction. Power-calibration was performed using a pulse-and-acquire sequence with a 6 cm distilled water phantom placed in the approximate coil region of maximum B1+ field. A B1+ field map was then acquired with 1.2x2.4x10 mm3 resolution throughout twelve coronal slices of a head phantom placed in the linear gradient region of the solenoid using the Bloch-Siegert B1+ technique[7] . A 6 ms Fermi pulse with KBS of 25.11 (ωRF = ±4 kHz) was used for the encoding pulse. Using the field map obtained through the center of the phantom and the Shinnar-Le Roux-based B1+-selective excitation pulse design algorithm described previously by our group[4], a nominal 30 mm thick axial slice offset from the phantom apex by 8 cm was excited in the phantom and an image acquired with a GRE sequence (128x67 matrix size, nominal flip angle = 45, 1.2x2.4 mm2 resolution, TE = 21 ms, TR = 430 ms). The same sequence was run with the B1+-selective excitation pulse replaced by typical B0 gradient-based slice encoding (excitation BW = 950 Hz, Gss = 22 mT/m). A diagram demonstrating the conversion between the two sequences is shown in Figure 2. All datasets were analyzed in Matlab. To verify slice-positioning, low-resolution 3D sequences for both encoding methods were also run by adding phase encoding partitions along the slice axis.
Results
The coil could be easily matched/tuned with power calibration indicating a 90° tip angle achieved using a 90 µs hard pulse with transmit power of 15.6 W. B1+ maps in Figure 3 indicate an approximately linearly decreasing transmit field throughout the head region ranging from 110-60 µT/A through a centerline in the phantom in the coil’s targeted 15 cm linear region. Results from the B1+-selective excitation experiment demonstrate slice selection using the coil with similar results obtained using either B1+ or B0-based slice selection as shown in Figures 4 and 5. Average SNR comparisons between the two datasets indicate a difference in average SNR of 15%, and images are relatively distortion-free.Discussion
Results demonstrate that B1+-based slice selection using the proposed system is feasible in vivo. The coil system performance matched predicted performance with similar results obtained through modeling and field mapping. Conversion of conventional encoding methods to RF encoding was straightforward and enabled slice encoding with comparable results to conventional slice selection.Conclusion
This work represents a first step in fully RF-encoded in vivo acquisition. To the authors’ knowledge, this is the first demonstration of a system appropriate for in vivo human brain imaging using B1+-selective excitation. Future work includes improving coil efficiency, in vivo imaging using the receive coil insert, and fabrication of additional RF systems to replace encoding in the remaining two spatial dimensions.Acknowledgements
The authors acknowledge financial support from NIH NIBIB Grant R01 EB 030414-01.References
[1] D I Hoult. Rotating frame zeugmatography. J Magn Reson, 33(1): 183-197, 1979.
[2] C J Hasselwander and W A Grissom. Bloch-Siegert phase-encoded MRI with a single RF coil and frequency-swept pulses. In Proceedings 25th Scientific Meeting, International Society for Magnetic Resonance in Medicine, Honolulu, 5047, 2017.
[3] Kartäusch R, Driessle T, Kampf T, et al. Spatial phase encoding exploiting the Bloch-Siegert shift effect. MAGMA, 27(5): 363-371, 2014. [3] Z Cao and W A Grissom. Frequency encoding by Bloch-Siegert shift. In Proceedings 22nd Scientific Meeting, International Society for Magnetic Resonance in Medicine, Milan, 4220, 2014.
[4] W A Grissom, Z Cao, and M P Does, |B1+|-selective excitation pulse design using the Shinnar-Le Roux algorithm. J Magn Reson, 242: 189-196, 2014.
[5] C LaPierre, M Sarracanie, D E J Waddington, and M S Rosen, A single channel spiral volume coil for in vivo imaging of the whole human brain at 6.5 mT. In Proceedings 23rd Scientific Meeting, International Society for Magnetic Resonance in Medicine, Toronto, 1793, 2015.
[6] Cooley, C.Z., McDaniel, P.C., Stockmann, J.P. et al. A portable scanner for magnetic resonance imaging of the brain. Nat Biomed Eng (2020). https://doi.org/10.1038/s41551-020-00641-5.
[7] L I Sacolick, F Wiesinger, I Hancu, and M K Vogel, B1 mapping by Bloch-Siegert shift. Magn Reson Med, 63(5): 1315–1322, 2010.
Figures