4031
Design of a portable, low-field magnetic resonance sensor for clinical measurement of volemic status1Health Science and Technology, Massachusetts Institute of Technology, Cambridge, MA, United States, 2Koch Institute for Integrative Cancer Research, Cambridge, MA, United States, 3Massachusetts Institute of Technology, Cambridge, MA, United States
Synopsis
A low-field portable MR-based sensor was designed for the acquisition of clinical T2 relaxometry measurements in skeletal muscle. A range of low-field permanent magnet array configurations were modeled with varying sensitive region depths and homogeneous volumes; an optimization score for each design was calculated. The optimal design has a sensitive region 15-20mm from the surface of the magnet, making it capable of acquiring measurements deep into the leg such that the measurement is fully localized to skeletal muscle. The exclusion of subcutaneous fat tissue in the sensitive region will improve sensitivity to fluid shifts within the skeletal muscle.
Background
Worldwide there are over 2.5 million patients with end stage kidney disease who depend on life-sustaining dialysis treatments. The intermittent nature of dialysis exposes patients to wide variations between fluid overload on non-dialysis days and fluid depletion after hemodialysis sessions1. Fluid overload has been linked with cardiovascular events and mortality2,3. Fluid depletion results in intradialytic hypotension, which occurs in up to 75% of hemodialysis patients and can cause nausea, vomiting, and cramping. Long-term volume overload and poorly managed ultrafiltration rates are also common4,5. There is currently no quantitative standard for fluid assessment that informs physicians or patients of their volemic status. An adequate assessment of fluid state in routine clinical practice is, therefore, an unmet need. There is no gold standard measurement for assessment of fluid volume status6. Methods for quantification of fluid status are known to have clinically relevant limitations7. We aim to establish the magnetic resonance (MR) based measurement as a rapid quantitative method for fluid assessment. We have developed a portable, single-sided MR sensor to collect relaxometry metrics from skeletal muscle, where excess fluid accumulates.We designed a portable single-sided MR relaxometer (SSR) that was tested in a dialysis patient study. Localized T2 relaxometry measurements were acquired from the lower leg of participants. Clinical MRI was able to able to detect differences between hyper- and hypo- volemic patients from lower leg skeletal muscle [FIG 1], but the SSR was unable to distinguish between those groups. SSR measurements did show statistically significant changes for each patient before and after hemodialysis. The SSR used in the study was designed with permanent magnets to have a static B0 magnetic field of 0.27 T. The magnet array is a Unilateral Linear Halbach design which is well suited for point of care settings because the magnetic field is limited to one side of the array. The sensitive saddle region of this sweet spot magnet is 2-7 mm from the surface of the magnet. Physically, this region includes both muscle and subcutaneous fat compartments when placed against a patient’s leg. The inclusion of subcutaneous fat in the measurement confounds the signal from the interstitial muscle space8 [Fig 2]. The purpose of this work is to design a portable permanent magnet sensor capable of acquiring signal from only the skeletal muscle of a leg and sufficiently sensitive enough to be used for clinical measurements.
Methods
The permanent magnet array was designed from ½” cube N-52 neodymium magnets for ease of modular fabrication9. The Unilateral Linear Halbach array and Cylindrical Halbach array magnet configurations are the basis for the design. These configurations allow for a sweet-spot homogeneous region above the surface of the magnet with few stray fields. Variations of magnet configurations, geometries, number of magnets in the x, y, and z directions and orientations of magnetization were explored. Finite element analysis was performed using COMSOL Multiphysics software to simulate the magnetic field for each design variation to identify the most robust sensor design to enable measurement of the muscle compartment. The design metric described in Greer et al.10 was used to determine optimal configuration that met the sweet spot depth requirements. This optimization score is shown in Equation 1 where G0 is static gradient, RMSE is the root mean square error of B0 field. $$Equation \ 1) \ \ \ Optimization\ Score = \frac{B_{0}^{7/4}}{RMSE * G_{0}}$$Results
A range of low-field permanent magnet array configurations were modeled in COMSOL with varying sensitive region depths and homogeneous areas, the optimization score for each design was calculated [Fig 3]. The design with the highest optimization score has a sensitive region 15-20mm from the surface of the magnet, making it capable of acquiring measurements deep into the leg such that the measurement is fully localized to skeletal muscle. The curved magnet geometry allows for a range of anatomic areas or tissue sample sizes to be placed in the sensitive region [Fig 4], and makes it more adaptable for patients with different body compositions. The sensitive region is defined by B0 field variation of less than 1%. The designed sensor can acquire data from a voxel size of 5cm3, over 50 times larger than the single-sided sensor used in the preliminary trial.Discussion
A low-field portable MR-based sensor was designed for the acquisition of clinical T2 relaxometry measurements in skeletal muscle. The exclusion of subcutaneous fat tissue in the sensitive region will improve sensitivity to fluid shifts within the skeletal muscle. The magnet is designed to seat the calf muscle, allowing for measurements deeper into the tissue, and reducing variability due to leg placement against a flat surface. Following fabrication, the sensor the B0 field will be mapped and the Shinnar-Le Roux algorithm will be used to design a B1 with a 50kHz bandwidth. The use of adiabatic pulses will allow for signal acquisition from a larger voxel size, which serves to increase the confidence in the clinical measurement of volumetric status in the tissue. This portable low-field magnet design will enable clinical assessment of fluid status without the need for full-scale clinical MRI imaging.Acknowledgements
We thank Dr. Matthew Rosen, Dr. Jason Stockmann, Chris Frangieh, and Ashvin Bashyam for helpful discussions and guidance.References
[1] Hecking M, Karaboyas A, Antlanger M, Saran R, Wizemann V, Chazot C, et al. Significance of interdialytic weight gain versus chronic volume overload: consensus opinion. Am J Nephrol. 2013;38(1):78–90
[2] Kalantar-Zadeh K, Regidor DL, Kovesdy CP, Van Wyck D, Bun- napradist S, Horwich TB, et al.: Fluid retention is associated with car- diovascular mortality in patients undergoing long-term hemodialysis. Circulation 119:671–679, 2009
[3] Wizemann V, Wabel P, Chamney P, Zaluska W, Moissl U, Rode C, et al.: The mortality risk of overhydration in haemodialysis patients. Nephrol Dial Transplant 24:1574–1579, 2009
[4] J.Q. Jaeger, R.L. Mehta, Assessment of dry weight in hemodialysis an overview, J. Am. Soc. Nephrol. 10 (1999) 392–403.
[5] J.E. Flythe, S.E. Kimmel, S.M. Brunelli, Rapid fluid removal during dialysis is associated with cardiovascular morbidity and mortality, Kidney Int. 79 (2011) 250–257.
[6] S. Ishibe, A.J. Peixoto, Methods of Assessment of Volume Status and Intercompartmental Fluid Shifts in Hemodialysis Patients: Implications in Clinical Practice, in: Semin. Dial., Wiley Online Library, 2004: pp. 37–43. doi:10.1111/j.1525-139X.2004.17112.x.
[7] S. McGee, W.B. Abernethy III, D.L. Simel, Is this patient hypovolemic?, Jama. 281 (1999) 1022–1029.
[8] L.Colucci, K. Corapi, M. Li, X. Parada, A Allegretti, H Lin, D. Ausiello, M. Rosen, M.J. Cima, Fluid assessment in dialysis patients by point-of-care magnetic relaxometry; Science Translational Medicine, 10.1126/scitranslmed.aau1749, Vol 11, Issue 502, 2019/07/24
[9] A. Bashyam, M. Li, M.J. Cima, Design and experimental validation of Unilateral Linear Halbach magnet arrays for singlesided magnetic resonance, J. Magn. Reson. (2018).
[10] M. Greer, C.Chen, S. Mandal, An easily reproducible, hand-held, single-sided, MRI sensor, Journal of Magnetic Resonance. 2019. Vol 308. ISSN 1090-7807.
Figures

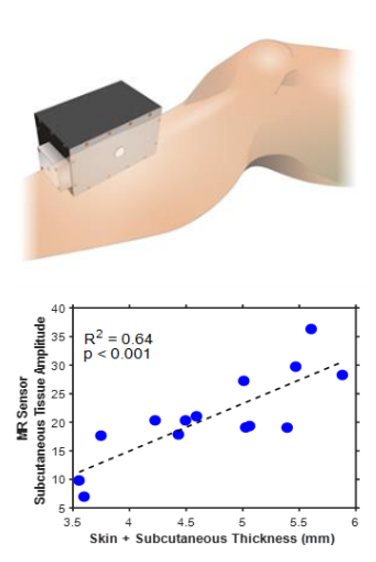
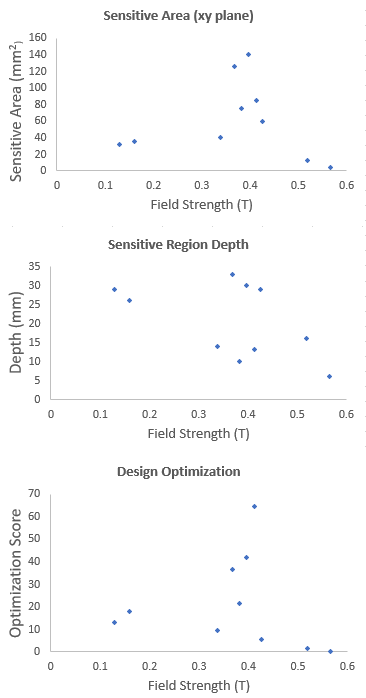
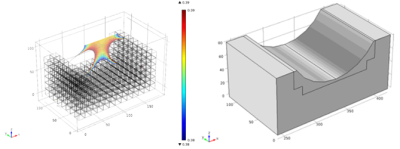
Figure 4. Selected magnet array design based on Unilateral Linear Halbach array and Cylindrical Halbach array configurations.