4029
A Novel Dedicated 0.35T Open Neonatal-Infant Brain MRI system1Department of Radiology, Children’s Hospital of Soochow University, Suzhou, China, 2Department of Radiology, The First People’s Hospital of Lianyungang, Jiangsu Province, China, 3Jiangsu LiCi Medical Device Co., Ltd, Lianyungang, China
Synopsis
A dedicated 0.35T Neonatal-Infant Brain MRI system is developed to investigate the effectiveness and safeness for neonatal and infant patients. 66 volunteers from 0-12 months are recruited to undergo a clinical trial and it is found that low field brain images displayed high grey-white tissue contrast, which is notably helpful for clinical diagnosis. The newly developed system also has very low acoustic noise and that most of the volunteer showed no sign of temperature raise. Low field MRI system therefore has the potential to be a effective and safer alternative MRI system for neonatal and infant patients.
Introduction
In the past many years, R&D in MRI technology has been concentrated in increasing the magnetic field strength. Although higher SNR and thinner slices with higher anatomical spatial details can be obtained with high field system, imaging sequences are mainly optimized for adult subject. However, at high field, for neonatal and infant with higher water content of the brain, notably the white matter, it makes the differential of gray and white matter difficult, which is further complicated at 3.0T and above because of the longer T1 relaxation times that already decrease T1 tissue contrast1. In view of this, we hypostasized that low field MRI system can be a effective and safer alternative system for neonatal and infant brain imaging and an investigation is undertaken. In this work, we designed and built a dedicated 0.35T Neonatal-Infant Brain MRI (NIB-MRI) system and performed a clinical trial with 66 volunteers from 0-12 months old. The results reported herein, showed that the acquired low field MR brain images can provide high clinical information and can be effectively used for medical diagnosis purposes. In addition, from the safety aspects it is shown to be a much safer system for neonatal and infant patients.Methods
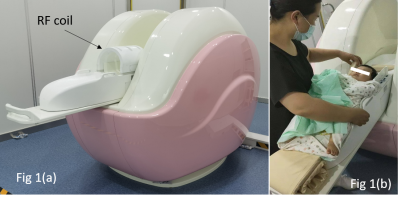
Shown in Fig 1 is the developed NIB-MRI system. A 0.35T open permanent magnet is used and positioned in a “U” shaped configuration. For minimizing SAR, a dedicated two channels transceive RF head coil is developed and optimized T1W-SE, T2W-FSE, T2W-FLAIR, DWI and 3D-T1-GRE pulse sequences for neonatal and infant brain imaging are also developed and tested. The NIB-MR system is firstly tested according to IEC 60601-1, 60601-1-2 and 60601-2-33 and passed all safety tests. 66 volunteers consisting of 33 male and 33 female subjects ranging from 0-12 months are recruited. A minimum 4 and maximum 7 subjects are allocated to each different month group (according to 12 different month groups). Ethical approvals for the clinical trial are given by the Children’s Hospital of SooChow University and The First People’s Hospital of Lianyungang ethics committee, and parental consent is obtained in all cases. During the clinical trial, 62 volunteers are administrated with chloral hydrate, while 4 volunteers are not given any form of sedation. All acquired images are independently scored based on MRI‐based five-point Likert scoring system by four experienced MR radiologists with above 10 years of experience in diagnosing low and high field MR images.Results
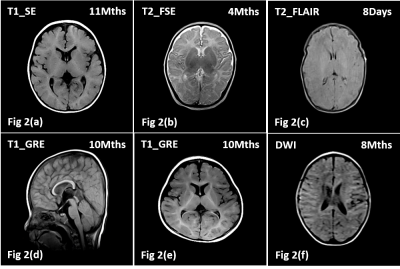
Shown in Fig 1(a) is the developed NIB-MRI system with the patient bed and the RF coil out. Fig 1(b) shows how the volunteer is positioned. The top part of the RF coil is first dislodged and the volunteer head is placed in the center of the RF coil, thereafter the top part of the RF coil is locked back into position and the bed is then pushed into the magnet. Shown in Figs 2(a-f) are the acquired brain images of the volunteers using our developed pulse sequence. Except for 3D-T1-GRE (Figs 2(d,e)), which used slice thickness of 2mm, the rest of the sequence used 5mm slice thickness.Discussion
Based on MRI‐based five-point Likert scoring system, it is found that 41 volunteers achieved a score of 5 (Maximum), 21 volunteers have a score of 4 and 4 volunteers are given a score of 3. Volunteers with score of 3 is due to movement of the subjects during the scan, which resulted in motion related artifacts. T1W and T2W images (Figs 2(a-e)) displayed high grey-white tissue contrast, which correlate well with the shorter T1 relaxation times that can be achieved with low field MRI system and is notably helpful for clinical diagnosis. The DWI is designed based on SE method without fat suppression and the B value is set as 600. Hence, high fat signal around the skull can still be observed as depicted in Fig 2(f). Acoustic noise of the NIB-MRI system is measured and the highest recorded noise level is ≤70dB(A). With ear protection, volunteer is subjected to ≤55dB(A) acoustic noise, which is close to the American Academy of Pediatrics recommended noise level of 45-50 dB(A)2. It should be noted that the 4 volunteers who are not sedated slept through their entire scan procedure and successfully completed their trial in a single attempt. For each volunteer, a pre and post scan body temperatures are measured and it is found that 47 volunteers showed no sign of temperature raise, 15 volunteers have <0.3°C raise and 4 volunteers have 0.3°C to 0.5°C raise, which are all well within ICNIRP recommendation of not exceeding 0.5°C for infant3.Conclusion
In this work, we have shown that brain images acquired with the developed 0.35T NIB-MRI system have high clinical value and can be effectively used for medical diagnosis. Initial findings on the safety aspects of the system has also been established and that the low-field NIB-MRI system has the potential to be a much safer system for neonatal-infant patients. Future work will concentrated on developing more efficient low field specific neonatal-infant brain imaging pulse sequence and further reducing the acoustic noise, dB/dt and SAR levels so as to provide a safer environment for neonatal and infant patients.Acknowledgements
No acknowledgement found.References
1. Tocchio S, Kline-Fath B, Kanal E, et al. MRI evaluation and safety in the developing brain. Seminars in Perinatology, 2015, 39(2):73-104.
2. American Academy of Pediatrics, Committee on Environmental Health. Noise: A Hazard for the Fetus and Newborn. Pediatrics, 1997, 100(4):724-727.
3. International Non-Ionizing Radiation Protection (ICNIRP). Statement of Medical Magnetic Resonance Procedures: Protection of Patients. Health Phys. 2004; 87(2):197–216.
Figures