4027
Feasibility of human in vivo skeletal muscle and cardiac proton spectroscopy at 0.75T1Institute for Biomedical Engineering, University and ETH Zurich, Zurich, Switzerland
Synopsis
Proton MR spectroscopy can assess cardiac triglyceride levels. Although spectral separation and thermal polarization decrease at lower field strength, increased T2* and T2 values, decreased T1 values, decreased echo times and the possibility to decrease receiver bandwidth are clear advantages compared to higher fields. In this work the feasibility of in vivo skeletal muscle and cardiac proton spectroscopy at 0.75T is demonstrated. For this purpose, a clinical 3T Philips Achieva scanner was ramped down to a field strength of 0.75T. Results are compared to spectra acquired at 1.5T and 3T and simulations confirm experimental findings.
Introduction
Proton MR spectroscopy has shown promising evidence to assess cardiac metabolism1. At the moment this is commonly performed at 1.5T or 3T. Higher field strengths have the advantage of higher thermal polarization and a larger peak separation for spectroscopy. This comes, however, at the expense of larger field inhomogeneities, higher SAR levels and increased ECG interference. Lower field strengths can benefit from lower SAR levels and therefore shorter RF pulse lengths and echo times2, which can together with increased T2 and decreased T1 values3–5 lead to an increase in signal. A more homogeneous field and therefore higher T2* values might compensate for the decrease in spectral separation at lower fields.The aim of the present work was to investigate the feasibility of 1H-MRS for the assessment of triglyceride levels in human calf muscle and myocardium at a custom 0.75T scanner and compare this to measurements at 1.5T and 3T.
Methods
A 3T Achieva scanner (Philips Healthcare, Best, the Netherlands) was ramped down to a field strength of 0.75T. A custom-made 4-channel transmit/receive coil array (Clinical MR Solutions, Shadybrook, WI, USA) (Figure 1), which was originally made to sample on the 13C frequency at 3T, was tuned to the 1H frequency at 0.75T. Reference measurements were performed at both a 1.5T Philips Achieva scanner using a five-channel cardiac receiver array and at another 3T Philips Achieva scanner using a two-channel Flex-L coil.Measurements were performed in both the calf muscle and the interventricular septum of 3 healthy volunteers. Spectra were acquired with a voxel size of 10×20×40 mm3; pencil-beam volume shimming and local power optimization6 were performed prior. TE ranged from 15 ms at 0.75T to 26 ms at 3T with a TR of 2000 ms. CHESS based water suppression was performed; the number of water-suppressed averages was 96 for calf muscle and 192 for myocardium. Spectral bandwidth in the leg was 1000 Hz, 2000 Hz and 4000 Hz at 0.75T, 1.5T and 3T respectively, and 2000 Hz in the heart for all field strengths (1024 samples). Cardiac measurements were ECG-triggered to end systole and respiratory gating was applied (window = 4 mm). Scan times were identical at all field strengths. Additionally, series with different echo times (range = 15-400 ms, 11 steps) and repetition times (range = 375-5000 ms, 9 steps) were acquired for the water-unsuppressed signal (NSA = 16) of the calf muscle.
All spectra were reconstructed in MATLAB using a customized reconstruction pipeline. Spectra were fitted with Lorentzian line shapes in the time-domain using AMARES (jMRUI, version 5.2)7. T1 was acquired by fitting the data from the TR series according to $$$M_z=M_0\left(1-c\left(e^{-\frac{TR}{T_1}}\right)\right)$$$, the TE series were fitted with $$$M_{xy}=M_0\left(e^{-\frac{TE}{T_2}}\right)$$$ and T2* was calculated based on linewidth of the water peaks according to $$$fwhm=\frac{1}{\pi T_{2}^{*}}$$$. T1, T2 and T2* were fitted as a function of magnetic field according to $$$T=a\left(B_0\right)^b$$$. Spectra consisting of TMA, CR, TG-CH2- and TG-CH3 were simulated using Bloch equations for field strengths of 0.75T, 1.5T and 3T. Realistic metabolite SNR values were assumed for 1.5T; SNR at 0.75T and 3T was calculated based on these values and an SNR simulation based on first principles. ΔB0 was calculated for each field strength based on the measured T2* of water in the septum; this was used to calculate T2* values of the separate metabolites and define linewidths.
Results
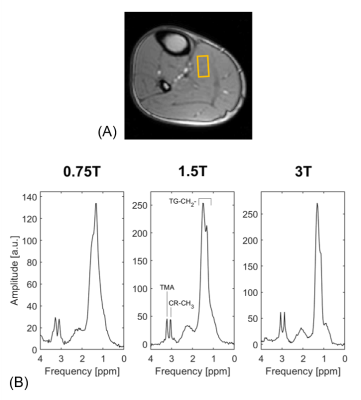
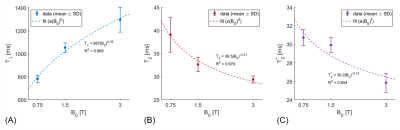
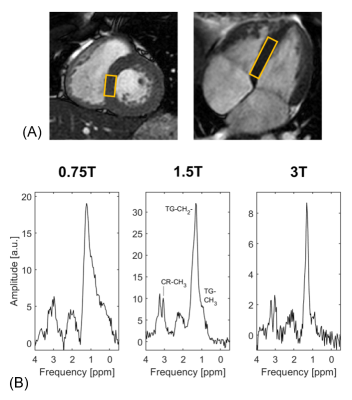
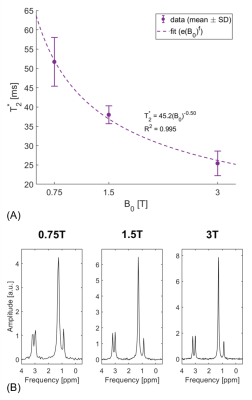
Exemplary spectra acquired from the soleus muscle from one volunteer at 0.75T, 1.5T and 3T are shown in Figure 2. Both triglycerides and creatine can be clearly distinguished in all three spectra. In Figure 3 the T1, T2 and T2* dependencies on field strength for the water signal of in vivo calf muscle are presented. T1 increases with increasing field strength, whereas both T2 and T2* decrease. Figure 4 compares exemplary spectra acquired in the interventricular septum of one subject at different field strengths. Even though peak separation decreases for lower field strengths, both TG and CR can be detected at 0.75T. Figure 5 shows T2* of myocardial water fitted as a function of magnetic field strength. This corresponds with linewidths of 6.22±0.74 Hz (0.75T), 8.40±0.51 Hz (1.5T) and 12.65±1.57 Hz (3T). Simulated spectra for different field strengths based on these T2* values, physiological metabolite relaxation parameters and realistic SNR values show that creatine can still be distinguished from TMA at 0.75T.Discussion
Although peak separation is reduced at low field, prolonged T2* makes spectral quality adequate to distinguish relevant metabolites. Decreased T1 values, increased T2 values, decreased echo times and the possibility to decrease receiver bandwidth can all lead to an increase in signal and are therefore clear advantages of lower fields. Chemical shift artefacts increase as a function of magnetic field strength, leading to better spatial localization at lower field strengths.The absence of a body coil for RF transmission at 0.75T was a limitation in this study; B1 power optimization was therefore challenging and the B1+ field was inhomogeneous. Local power optimization could, however, be performed for the spectroscopic measurements.
Conclusion
Both measurements and simulations using first principles indicate that human in vivo proton spectroscopy in skeletal muscle and in the heart is achievable at 0.75T.Acknowledgements
No acknowledgement found.References
1. van Ewijk PA, Schrauwen-Hinderling VB, Bekkers SCAM, Glatz JFC, Wildberger JE, Kooi ME. MRS: a noninvasive window into cardiac metabolism. NMR Biomed. 2015;28:747–766.
2. Marques JP, Simonis FFJ, Webb AG. Low-Field MRI: An MR Physics Perspective. J. Magn. Reson. Imaging 2019;49:1528–1542.
3. Bottomley PA, Foster TH, Argersinger RE, Pfeifer LM. A review of normal tissue hydrogen NMR relaxation times and relaxation mechanisms from 1-100 MHz: Dependence on tissue type, NMR frequency, temperature, species, excision, and age. Med. Phys. 1984;11:425–448.
4. Campbell-Washburn AE, Ramasawmy R, Restivo MC, et al. Opportunities in Interventional and Diagnostic Imaging by Using High-performance Low-Field-Strength MRI. Radiology 2019;293:384–393.
5. Krššák M, Lindeboom L, Schrauwen-Hinderling V, et al. Proton magnetic resonance spectroscopy in skeletal muscle: Experts’ consensus recommendations. NMR Biomed. 2020;e4266.
6. de Heer P, Bizino MB, Lamb HJ, Webb AG. Parameter Optimization for Reproducible Cardiac 1H-MR Spectroscopy at 3 Tesla. J. Magn. Reson. Imaging 2016;44:1151–1158.
7. Vanhamme L, van den Boogaart A, Van Huffel S. Improved Method for Accurate and Efficient Quantification of MRS Data with Use of Prior Knowledge. J. Magn. Reson. 1997;129:35–43.
Figures