4022
Magnetic Resonance Guided Percutaneous Cryoablation of Extra-abdominal Desmoid Tumors: An Institutional Experience1Radiology, Stanford University, Palo Alto, CA, United States
Synopsis
Desmoid tumors are challenging to control with chemotherapy, surgery, or radiation. This retrospective review evaluated patients with extra-abdominal desmoid tumors treated with MR-guided cryoablation at our institution over two years. The primary endpoint was tumor volume change before and 3 months after cryoablation; the secondary endpoint was the change in health status assessed at the same interval. Our study demonstrates MR-guided cryoablation results in the reduction of both tumor size and improvement in health status scores. No severe adverse events occurred. We conclude that MR-guided cryoablation is an effective and safe treatment option for desmoid tumors.
Introduction
Desmoid tumors are rare monoclonal tumors consisting of spindle fibrocyte-like cells that form along the musculoaponeurotic structures1. These tumors lack metastatic potential but can cause significant morbidity from local infiltration around nerves, vessels, and muscles. Current treatment strategies include observation2-4, surgical resection, radiation, systemic therapy, high intensity focused ultrasound5,6, and percutaneous thermal ablation (radiofrequency ablation and cryoablation)7-10. While initially used primarily in the salvage setting, the 2020 treatment guidelines from the National Comprehensive Cancer Network (NCCN) lists ablation as a first-line therapeutic option for extra-abdominal desmoid tumors11.This study investigates our initial two-year experience with MR-guided cryoablation treatment of extra-abdominal desmoid tumors.
Methods
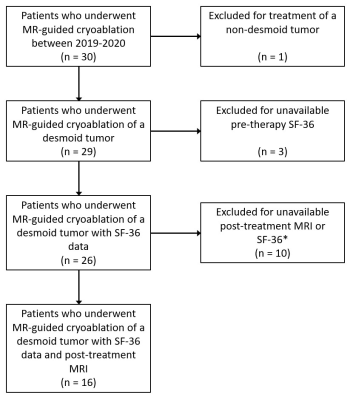
Patient SelectionAfter approval from our institutional review board, we retrospectively reviewed patients with biopsy-proven desmoid tumors treated with MRI-guided percutaneous cryoablation between 2019-2020 (Figure 1). All patients underwent multi-disciplinary evaluation. Patients had pre-procedure and 3-month post-treatment MR imaging.
Data Collection
Data recorded through a retrospective chart review included demographic patient information, prior treatments, symptoms, lesion volume, therapy specifications, and complications.
Pre-ablation and three-month post-ablation assessment compared 1) pre- and post-ablation viable tumor volume measured by contouring the tumor on subtracted post-contrast MRI, and 2) changes in health status based on the 36-item short-form questionnaire (SF-36)12. Complications were recorded per the Society of Interventional Radiology Adverse Event Severity Scale13.
Cryoablation Technique
After informed consent, general anesthesia was induced. Angiocatheters (14g Becton Dickinson) were advanced into the tumor under ultrasound- and MRI-guidance for tumor penetration. Placement of cryoprobes (Visual ICE Cryoablation System, Boston Scientific, USA) at the appropriate depth through angiocatheters were confirmed with MRI. The radiologist determined the number of probes based on the targeted tumor size (median: 6; range: 2 – 16). Hydro-dissection was performed under ultrasound- and MRI-guidance to ensure the safety of the adjacent structures. Measures for skin protection included warming packs, warmed ultrasound gel, and warmed saline.
The number (median: 4 cycles; range 2 – 6 cycles) and duration (median: 8 minutes, range 3.5 – 15 minutes) of freeze-thaw cycles varied based on tumor size and proximity to critical structures. The goal was to extend the ice ball 5 mm beyond the tumor margin while avoiding critical structures. During all cryoablation cycles, ice ball proximity to critical structures was monitored with intermittent MR imaging (Figure 2). Patients were discharged or admitted for observation depending on the complexity of the procedure and medical comorbidities. Complications were assessed immediately after, 1-day, 2-days, 1-week, and 3-months following the procedure. Post-cryoablation MRI was performed at the 3-month interval.
Results
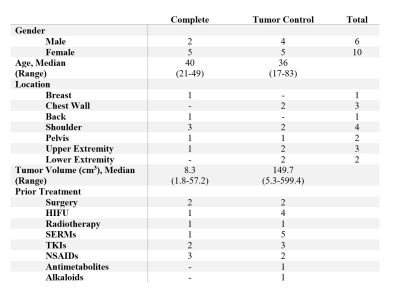
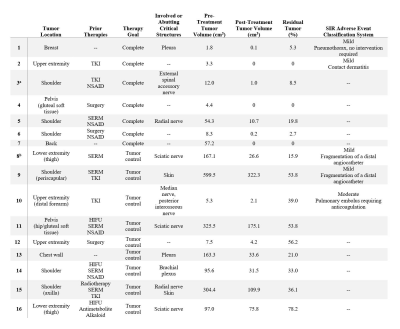
A total of 16 patients (median age: 40, range 17 – 83; 10 females) received 18 MR-guided percutaneous cryoablation procedures (Table 1). Two patients required repeat procedures due to tumor size. Lesions were in the extremities, shoulder, chest wall, pelvis, back, and breast. The median pretreatment tumor volume was 56 cm3 (mean: 119 cm3, range: 2 – 600 cm3) (Table 2).In seven patients, complete ablation was the pre-treatment goal. The mean residual tumor volume was 5.2% at follow up (range 0 – 19.8%). Two adverse events occurred in this subgroup: a small pneumothorax, which required no intervention, and contact dermatitis, possibly from adhesive tape. All patients had improved health status scores following cryoablation (Table 3).
Subtotal cryoablation was performed in nine patients due to the proximity to critical structures, with the intent to control tumor size and symptoms. The mean residual tumor volume was 42.9% at follow-up (range 15.9-78.2%). Fragmentation of the angiocatheter tip occurred in two patients while one patient had a post-procedure pulmonary embolism. Changes in health status scores were relatively small and inconsistent (Table 3).
Discussion
Our study demonstrates the safety and efficacy of MR guided cryoablation of desmoid tumors. At three-month follow-up, all patients had reduced viable tumor volumes, including three patients with no residual tumor. Although endpoints vary and our follow-up is shorter, our outcomes are encouraging when compared with medical approaches to management, which have reported measurable response rates (decreased tumor diameter) of 31%14 and 33%15 at 12 months or lack of progression rates at 6 months between 45 – 83.7%16. Patients with the intention of complete ablation reported improved health status scores on all components of the SF-36 questionnaire. Patients treated with the intention of tumor control reported little change in health status; it is possible for some patients that the same proximity to nerves that limited total ablation allowed for ongoing symptoms despite the reduction in overall tumor volume.Our mean decrease in viable tumor volume of 73.6% at 3 months is similar to prior studies. Redifer Tremblay et al reported a mean 81% decrease in viable tumor volume at 16.8 months in 23 desmoid patients who underwent cryoablation; tumor size (mean:114.8 cm, range: 0.4 – 456.5 cm3) was similar to our cohort10. Schmitz et al reported a mean 70% decrease in viable tumor volume in 18 patients with a mean tumor size of 38.1 cm3 (1.6 – 118.3 cm3)9, while Havez et al reported a mean 87% decrease in viable tumor volume in 13 patients8.
Limitations of the study include small sample size, single-institution experience, and short follow-up.
Conclusion
MR-guided cryoablation is a safe and efficacious treatment strategy for extra-abdominal desmoid tumors.Acknowledgements
No acknowledgement found.References
1. Alman BA, Pajerski ME, Diaz-Cano S, Wolfe HJ. Aggressive fibromatosis (desmoid tumor) is A. Diagn Mol Pathol. 1997;6(2):98-101.
2. Bonvalot S, Ternès N, Fiore M, et al. Spontaneous regression of primary abdominal wall desmoid tumors: More common than previously thought. Annals of Surgical Oncology. 2013;20(13):4096-4102.
3. Eastley N, McCulloch T, Esler C, et al. Extra-abdominal desmoid fibromatosis: A review of management, current guidance and unanswered questions. European Journal of Surgical Oncology (EJSO). 2016;42(7):1071-1083.
4. Colombo C, Miceli R, Le Péchoux C, et al. Sporadic extra abdominal wall desmoid-type fibromatosis: Surgical resection can be safely limited to a minority of patients. Eur J Cancer. 2015;51(2):186-192.
5. Ghanouni P, Dobrotwir A, Bazzocchi A, et al. Magnetic resonance-guided focused ultrasound treatment of extra-abdominal desmoid tumors: A retrospective multicenter study. Eur Radiol. 2017;27(2):732-740.
6. Wang Y, Wang W, Tang J. Ultrasound-guided high intensity focused ultrasound treatment for extra-abdominal desmoid tumours: Preliminary results. International Journal of Hyperthermia. 2011;27(7):648-653.
7. Kurtz J, Buy X, Deschamps F, et al. CRYODESMO-O1: A prospective, open phase II study of cryoablation in desmoid tumour patients progressing after medical treatment. Eur J Cancer. 2020;143:78-87.
8. Havez M, Lippa N, Al-Ammari S, et al. Percutaneous image-guided cryoablation in inoperable extra-abdominal desmoid tumors: A study of tolerability and efficacy. Cardiovasc Intervent Radiol. 2014;37(6):1500-1506.
9. Schmitz JJ, Schmit GD, Atwell TD, et al. Percutaneous cryoablation of extraabdominal desmoid tumors: A 10-year experience. Am J Roentgenol. 2016;207(1):190-195.
10. Redifer Tremblay K, Lea WB, Neilson JC, King DM, Tutton SM. Percutaneous cryoablation for the treatment of extra‐abdominal desmoid tumors. J Surg Oncol. 2019;120(3):366-375.
11. National Comprehensive Cancer Network. Clinical practice guidelines in oncology (NCCN guidelines) soft tissue sarcoma version 2. 2019. Im Internet: www.nccn.org. 2020.
12. Ware Jr JE. SF-36 health survey update. Spine. 2000;25(24):3130-3139.
13. Khalilzadeh O, Baerlocher MO, Shyn PB, et al. Proposal of a new adverse event classification by the society of interventional radiology standards of practice committee. Journal of Vascular and Interventional Radiology. 2017;28(10):1432-1437. e3.
14. Skapek SX, Ferguson WS, Granowetter L, et al. Vinblastine and methotrexate for desmoid fibromatosis in children: Results of a pediatric oncology group phase II trial. Journal of Clinical Oncology. 2007;25(5):501-506.
15. Gounder MM, Mahoney MR, Van Tine BA, et al. Sorafenib for advanced and refractory desmoid tumors. N Engl J Med. 2018;379(25):2417-2428.
16. Toulmonde M, Pulido M, Ray-Coquard I, et al. Pazopanib or methotrexate–vinblastine combination chemotherapy in adult patients with progressive desmoid tumours (DESMOPAZ): A non-comparative, randomised, open-label, multicentre, phase 2 study. The Lancet Oncology. 2019;20(9):1263-1272.
Figures
Complete (top): Pre-ablation post-contrast axial T1WI (a) with a desmoid tumor (arrows) in right arm. Intraprocedural MRI (b) depicts the ice-ball (*) in the tumor. Post-ablation post-contrast T1WI (c) shows the ablation cavity (dotted arrows) without residual tumor.
Tumor control (bottom): Pre-ablation post-contrast axial T1WI (d) with a periscapular desmoid tumor (arrows). Intraprocedural MRI (e) shows the ice-ball (*) with repositioning of cryoprobes (f). Post-cryoablation post-contrast T1WI (g) shows nonviable tumor (dotted arrows) and residual tumor (arrows).