4016
MR SIGnature MAtching (MRSIGMA) with retrospective self-validation for real-time volumetric tumor motion imaging1Medical Physics, Memorial Sloan Kettering Cancer Center, New York, NY, United States, 2Radiation Oncology, Memorial Sloan Kettering Cancer Center, New York, NY, United States, 3Radiology, Memorial Sloan Kettering Cancer Center, New York, NY, United States
Synopsis
MRSIGMA is a promising real-time volumetric imaging for MRI-guided adaptive radiotherapy using a MR-Linac system. However, the lack of a real-time 3D reference image acquired with similar temporal resolution introduces significant challenges for in vivo validation. This work proposes a retrospective self-validation for MRSIGMA, where the same data used for real-time imaging are used to create a non-real-time reference. MRSIGMA with self-validation is tested in patients with liver tumors using quantitative metrics defined on the tumor and nearby organs-at-risk structures.
Introduction
The MR-linac, which combines an MRI scanner and a linear accelerator, provides a platform for MRI-guided adaptive radiotherapy of moving organs1,2. For example, the shape of the radiation beam can be adapted in real-time to the tumor motion using a multi-leaf collimator(MLC)3,4. However, current real-time MRI technology in the MR-linac is limited to 2D imaging. MR SIGnature MAtching (MRSIGMA) was recently introduced for real-time 3D imaging in the context of the MR-linac, consisting of a non-real-time learning step to compute pairs of 3D motion states and signatures and a real-time signature matching step to rapidly acquire signature data only and perform signature matching. MRSIGMA was able to achieve approximately 250ms of total imaging latency (including acquisition and reconstruction)5. However, MRSIGMA was only validated qualitatively without a proper reference. This work presents a novel version of MRSIGMA with retrospective self-validation, where the same data used for real-time signature matching are used to reconstruct a non-real-time 4D reference for comparison. MRSIGMA with retrospective self-validation was tested on patients with liver tumors referred for radiotherapy.Methods
MRSIGMA: The offline learning step (non-real-time) uses the XD-GRASP approach6 to create the database of 3D motion states and corresponding motion signatures (Figure 1.a). First, a motion signal is estimated from the center of k-space. Second, the motion signal is binned into motion signatures, where the signature for each motion state is the motion amplitude range of each respiratory bin. Third, data is sorted into undersampled 3D motion states. Fourth, temporal compressed sensing reconstruction is performed to obtain unaliased 3D motion states. A total of 10 motion states were computed in this work. The online matching step (real-time) uses the same acquisition trajectory, but each radial line is immediately processed to compute a motion signature and to find a match in the pre-computed database of 10 3D motion states (Figure 1.b).Retrospective self-validation: Since data acquisition during offline training and online matching are similar, this works proposes to use a XD-GRASP reconstruction using all the data acquired during online matching to retrospectively form a 4D reference (10 motion states in this work) with similar temporal resolution to MRSIGMA. The 4D reference image was computed by retrospectively assigning one of the XD-GRASP motion states to each spoke during online matching (Figure 2).
Data Acquisition: Data was acquired on a 3T MRI scanner (Philips Ingenia,Best,The Netherlands) using the 3DVANE pulse sequence in research mode to enable golden-angle stack-of-stars acquisition. Three patients with liver tumors participated in the study.
Image Reconstruction: Real-time MRSIGMA and non-real-time XD-GRASP reference images were reconstructed in MATLAB (MathWorks,Natick,MA) using in-house algorithms and raw data imported from the scanner. Images were converted to dicoms for evaluation.
Image contouring: Tumors and nearby organs-of-interest (liver, kidneys, and pancreas) in each motion state of the MRSIGMA database and reference database were manually contoured by a radiation oncologist. The contours were performed in MIM (MIM Software Inc.) on individual axial slices, which were later interpolated to obtain 3D contours.
Quantitative evaluation: The dice coefficient7 was employed to quantitatively compare the contours corresponding to MRSIGMA and the reference for each time point. The dice coefficient is given by $$$d(A,B)=\frac{2\times Area(Intersection(A,B))}{Area(A)+Area(B)}$$$, where A and B are the contours being compared and is 1 if they completely overlap.
Results
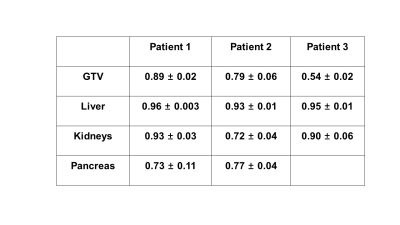
Figure 3 shows real-time 3D motion imaging of the liver tumor and organs of interest for the first patient and Figure 4 shows the dice coefficient over time for the same patient. The mean value ± standard deviation of the dice coefficient for the tumor, liver, kidneys, and pancreas were 0.89±0.02, 0.96±0.003, 0.93±0.03, and 0.73±0.11 respectively. The mean value of the dice coefficient was close to 1 and the standard deviation was lower than 0.03 for the liver tumor, liver and kidneys, which demonstrate high performance real-time 3D motion imaging in these anatomical regions. The lower performance in the pancreas is mainly due to the reduced T1-weighting contrast which limited ability to contour. Table 1 presents the mean±standard deviation of the dice coefficient for all three patients, where patients 2 and 3 show similar trends in organs-at-risk contours. The dice coefficient corresponding to the GTV for patient 3 is the lowest because the tumor was not clearly visible even on the conventional NUFFT reconstruction (no motion sorting). Future work will consider the utilization of a gadolinium-based contrast agent to enhance GTV visualization in T1w images.Discussion
The retrospective self-validation approach for MRSIGMA provides a mechanism to quantitatively evaluate real-time volumetric motion imaging performance in each study. The mean value and standard deviation of the dice coefficient over time can be used to assess motion imaging accuracy and uncertainty during the real-time imaging period. The utilization of the same data increases robustness of the validation and removes potential inconsistencies in using a separate reference. In addition, real-time adaptation to 3D shapes enables real-time dose reconstruction of mobile organs on the MR-linac.Conclusion
This study demonstrated that real-time 3D imaging results of MRSIGMA can be validated using a reference estimated from the same data used for signature matching. The quantitative self-validation can be used as a metric for real-time motion imaging performance, a current need in clinical real-time imaging.Acknowledgements
No acknowledgement found.References
1. Mutic S, D.J., The ViewRay system: magnetic resonance‐guided and controlled radiotherapy. Semin Radiat Oncol, 2014. 24: p. 196-199.
2. Raaymakers BW, L.J., Overweg J, et al, Integrating a 1.5 T MRI scanner with a 6 MV accelerator: proof of concept. Phys Med Biol, 2009. 54: p. N229-N237
3. Keall PJ, K.V., Vedam SS, Mohan R, Motion adaptive x-ray therapy: a feasibility study. Phys Med Biol, 2001. 46(1).
4. Poulsen PR, C.B., Sawant A, Keall PJ, Implementation of a new method for dynamic multileaf collimator tracking of prostate motion in arc radiotherapy using a single kV imager. Int J Radiation Oncol Biol Phys, 2010. 76(3): p. 914-923.
5. Feng L, T.N., Otazo R, MRSIGMA: Magnetic Resonance SIGnature MAtching for real-time volumetric imaging. Magnetic Resonance in Medicine, 2020. 84(3): p. 1280-1292.
6. Feng L, A.L., Chandarana H, Block KT, Sodickson DK, Otazo R, XD-GRASP: Golden-angle radial MRI with reconstruction of extra motion-state dimensions using compressed sensing. Magn Reson Med, 2016. 75: p. 775-788.
7. Taha AA, H.A., Metrics for evaluating 3D medical image segmentation: analysis, selection, and tool. BMC Med Imaging, 2015. 15.
Figures