4015
Clinical evaluation of 4DMRI for lung cancer radiation treatment planning1Calvary Mater Hospital, Newcastle, Australia, 2Univeristy of Newcastle, Newcastle, Australia, 3Blacktown Cancer & Haematology Centre, Blacktown, Australia
Synopsis
4D-CT is routinely acquired for lung cancer treatment planning to visualise the extent of tumour motion, to determine the appropriate treatment target volume. However, it can be unreliable in cases of irregular breathing, is susceptible to image artefact in regions close to the diaphragm, and shows generally poor soft tissue contrast. In this study we evaluated an alternative self-navigating 4D-MRI approach in terms of motion detection accuracy, measured tumour volume, and dosimetric differences observed with respect to patients’ existing lung cancer treatment plans.
INTRODUCTION
4D-CT is acquired for lung cancer treatment planning for visualising the extent of tumour motion. Acquired image data is retrospectively binned into respiratory phases using a respiratory trace signal. Accurate image binning requires a regular a breathing pattern to avoid artefact and misrepresent tumour position. Additionally, poor soft tissue contrast in CT may lead to miss-identification of tumour with surrounding tissue 1-3. MRI by comparison, has demonstrated superior soft tissue contrast with improved definition of tumour extent 4, while 4D-MRI has demonstrated improved diagnostic value for moving anatomy 5-7. In this work we investigated the viability of 4D-MRI for lung cancer treatment planning and compare dosimetric outcome in context with patients’ existing treatment based plans.METHODS
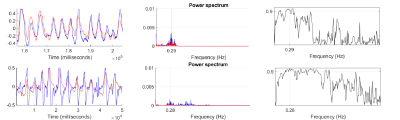
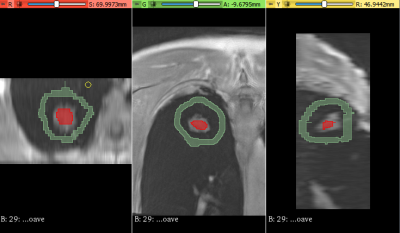
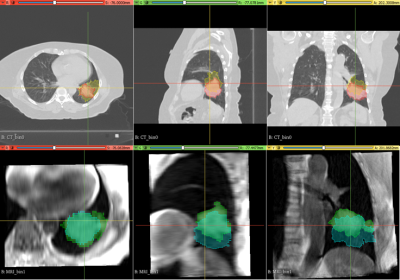
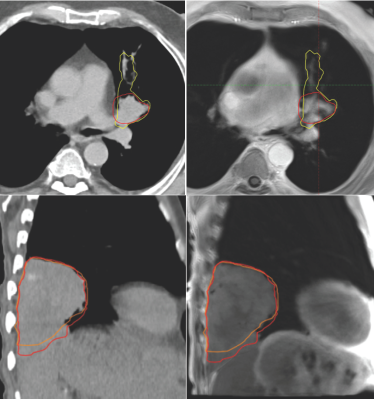
10 lung cancer patients scheduled for radiation treatment planning, were recruited as part of an ethically approved clinical study. In addition to 3D and 4D-CT used for standard treatment planning, patients also underwent an MRI session. This included a prototype self-navigating radial Volume Interpolated Breath hold Exam (VIBE) with motion correction (Siemens), to generate 4D-MRI data sets during free breathing after retrospective phase binning of respiratory motion. Transverse average and Coronal imaging with self-navigation was acquired: 3000 radial spokes, 0.9×0.9×3 mm resolution, with retrospective motion correction averaging and respiratory phase binning, generating 5 image volumes ranging from end-exhalation to end-inhalation. A respiratory bellows sensor was also fitted to patients during scanning to provide a reference breathing pattern. To assess motion detection accuracy of the radial VIBE sequence, self-navigation and bellows traces were analysed in Matlab to determine the level of coherence. A 3D slicer tool 8, 9 was used to segment tumour volumes in both 4D-CT and 4D-MRI image bins to quantify the extent of superior – inferior tumour motion (Fig.2) and to assess possible hysteresis related volume changes. Sørensen–Dice index and DICE co-efficient were calculated to assess the spatial overlap of 4D-CT and 4D-MRI planning treatment volumes (PTV). 4D-MRI and 3D-CT were used by three radiation oncologists, to contour internal treatment volumes and derive the subsequent PTV using Eclipse treatment planning system (Varian). These new structures were imported into the patients’ original treatment plan to assess dosimetric differences associated with the use of 4D-MRI.RESULTS
Evaluation of self-navigation and bellows signals across 10 patients measured during 4D-MRI, demonstrated a high coherence estimate (0.97±0.01). Figure 1 show examples of good correlation and poor correlation between self-navigation file and respiratory bellows trace. Analysis of 4D-CT and 4D-MRI tumour volumes at both end-inhalation and end-exhalation showed high correlation (r=0.99, p<0.0001), suggesting a linear relationship between the two methods (Fig. 3). Paired t-test between CT and MRI showed that the measured tumour volume size were smaller in MRI than CT (averageCTinhale = 125[cm3], averageMRinhale = 111 [cm3], paired t-test pinhale<0.04, averageCTexhale = 126[cm3], averageMRexhale = 103 [cm3], paired t-test pexhale< 0.04 ). These result suggest MRI may have clearer contrast between tumour and surrounding tissue. Additionally, differences in tumour volume between CT (bins 0 - 4) and MRI (bins 1-5) were assessed. A paired t-test showed no significant differences between the two modalities (p >0.05) with respect to change in volume. Paddick conformance index of 95% isodose volume and PTV for MRI and CT showed good correlation (r=0.8, P<0.005), while the mean Sørensen–Dice index for MRI and CT PTV similarity across all subjects was 0.86±0.12. Dose difference > 2% was observed between MRI and CT PTV D95% in 8 out of 10 patients with the existing patient treatment plan.CONCLUSION
4D–MRI in lung cancer patients, demonstrated a high level of accuracy in motion detection in most patients. MRI tumour volumes were typically smaller than the equivalent CT volumes, and showed improved tissue contrast. Clear differences were observed in dose outcome when assessed with the existing radiation treatment plan. Further work is required to assess the impact of treatment re-planning with MRI data, and to further identify the limitations 4D-MRI for lung cancer treatment planning.Acknowledgements
Jianing Pang (Siemens, Healthineers) for development of and assistance with the use of the prototype pulse sequence - Radial VIBE with Self-Gating and Motion Correction.
Daniel Staeb (Siemens, Healthineers) for assistance with respiratory bellows log file interpretation.
References
1. Werner, R., et al. Reduction of breathing irregularity-related motion artifacts in low-pitch spiral 4D CT by optimized projection binning.(Report). Radiat Oncol. 2017;12(1), https://doi.org/10.1186/s13014-017-0835-7.
2. Pollock, S., et al. The impact of breathing guidance and prospective gating during thoracic 4DCT imaging: an XCAT study utilizing lung cancer patient motion. Phys Med Biol. 2016;61(17):6485-6501, https://doi.org/10.1088/0031-9155/61/17/6485.
3. Brandner, E.D., et al. Motion management strategies and technical issues associated with stereotactic body radiotherapy of thoracic and upper abdominal tumors: A review from NRG oncology. Med Phys. 2017;44(6):2595-2612, https://doi.org/10.1002/mp.12227.
4. Chandarana, H., et al. Emerging role of MRI in radiation therapy. J Magn Reson Im. 2018;48(6):1468-1478, https://doi.org/10.1002/jmri.26271.
5. Kolbitsch, C., et al. Cardiac and Respiratory Motion Correction for Simultaneous Cardiac PET/MR. J. Nucl. Med. 2017;58(5):846, https://doi.org/10.2967/jnumed.115.171728.
6. Robson, P.M., et al. Correction of respiratory and cardiac motion in cardiac PET/MR using MR-based motion modeling. Phys Med Biol. 2018;63(22):225011-225011, https://doi.org/10.1088/1361-6560/aaea97.
7. Deng, Z., et al. A post-processing method based on interphase motion correction and averaging to improve image quality of 4D magnetic resonance imaging: a clinical feasibility study. Brit J. Radiol. 2019;92(1095):20180424-20180424, https://doi.org/10.1259/bjr.20180424.
8. Fedorov A., Beichel R., Kalpathy-Cramer J., et al. 3D Slicer as an Image Computing Platform for the Quantitative Imaging Network. Magnetic Resonance Imaging. 2012 Nov;30(9):1323-41. PMID: 22770690. https://doi.org/10.1016/j.mri.2012.05.001
9. Velazquez E, Parmar C, Jermouni M, Mal R et al. Volumetric CT-based segmentation of NSCLC using 3D-Slicer. Sci Rep. 2013 Dec 18;3:3529. https://doi.org/10.1038/srep03529
Figures