4014
Feasibility of free breathing real-time cine-MRI for MR-guided Cardiac Radioablation on the Unity MR-linac1Radiotherapy, University Medical Center Utrecht, Utrecht, Netherlands, 2Philips Healthcare, Best, Netherlands
Synopsis
Stereotactic arrhythmia radioablation (STAR) is a novel non-invasive treatment technique for ventricular tachycardia patients, refractory to conventional catheter ablation. Here, we investigate the feasibility of integrating real-time, free-breathing, cardiac cine-MRI into the STAR radiotherapy workflow. As a first step towards MRI-guided STAR treatments, we test our imaging approach on a diagnostic 1.5T MRI-simulator (Philips, Ingenia), and on a 1.5T Elekta Unity MR-linac. Our results indicate the feasibility of acquiring real-time, free-breathing, cine MRI images on both systems at 14Hz, enabling real-time motion assessment and delivery adaptation without additional cardiac imaging hardware.
Introduction
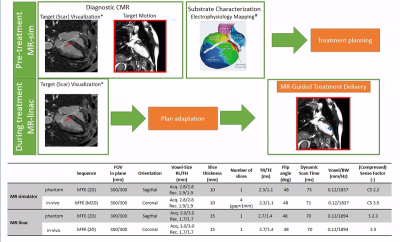
Stereotactic arrhythmia radioablation (STAR) is a novel non-invasive treatment technique for ventricular tachycardia patients. During STAR, a single ablative radiotherapy dose (25Gy) is delivered to the arrhythmogenic scar region. Among other benefits, STAR allows accessing locations that are unreachable for catheter ablation (e.g., intramural scars). Recent STAR case series show a remarkable (>85%) reduction in VT burden for refractory patients(1,2). One of the key requirements of STAR is the precise dose delivery to a small target within the left ventricle (LV) that is subject to both respiratory and cardiac motion. We hypothesize that both types of motion can be simultaneously monitored and mitigated in real-time on the novel hybrid 1.5T MRI-linac. To this end, we propose an MRI-guided STAR treatment workflow (Fig.1) to quantify motion during treatment simulation, and to adapt the treatment delivery in real-time(3). In this study, we investigate the feasibility of acquiring real-time (RTE), free-breathing, cardiac cine-MRI as preparatory work for STAR treatment planning and delivery on MR-linacs.Methods
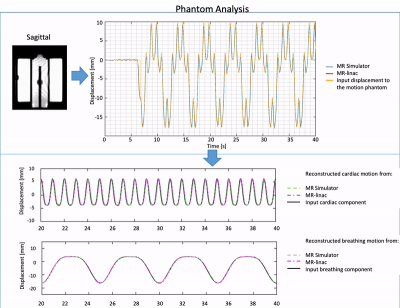
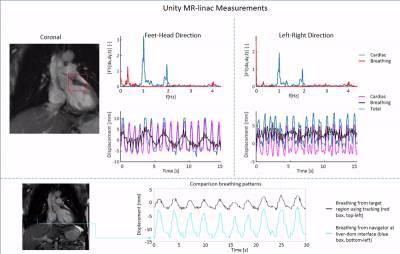
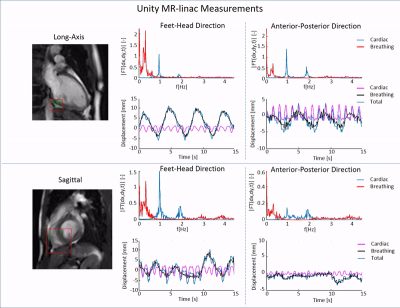
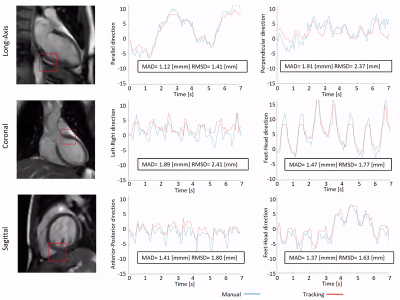
First, feasibility of the RTE, cine-MRI approach was validated using an MR-compatible 4D motion phantom (ModusQA, Ontario, Canada), which allowed moving a spherical target according to a pre-programmed trajectory (ground-truth): sum of two cos4 waveforms mimicking cardiac (60bpm, 10mm peak-to-peak amplitude) and respiratory (12bpm, 20mm peak-to-peak amplitude) motion. The target motion was estimated using circle Hough transformation on images acquired on a diagnostic 1.5T MRI-simulator (Ingenia, Philips, Best, Netherlands) and a 1.5T Unity MR-linac (Elekta AB, Stockholm, Sweden). RTE cine-MRI parameters are shown in Fig.1. During post-processing, we separated the respiratory and cardiac motion components using a bandpass filter and we compared them with the ground-truth input waveform for validation. Second, the feasibility of the RTE, free-breathing, cardiac cine-MRI approach was tested on a healthy volunteer (male, 26y). MR images were acquired coronally, sagittally, and along the cardiac long-axis. Automated target tracking was performed for all acquisitions by means of template-matching using normalized cross-correlation in a 6x6 cm2 search region. Prior to template-matching, images were median-filtered (3x3) and upsampled (x2). For the MR-linac acquisitions, the resulting total motion signal was deconvoluted into cardiac and respiratory components as was done for the phantom data. For the MR-simulator acquisitions, the automatically extracted motion was compared to manually annotated landmark locations in all dynamics by calculating the mean-absolute-deviation (MAD) and the root-mean-squared-deviation (RMSD). Various landmarks in the LV wall (Fig.5, red boxes) were selected for the different orientations to demonstrate the flexibility of the proposed motion estimation.Results
The phantom reconstructions (Fig.2) demonstrate the feasibility of extracting cardiac and respiratory motion components from the acquired RTE cine-MR images. The estimated motion components agree well with the ground-truth motion trajectories. The good contrast between myocardial wall and blood pool in-vivo allowed for reliable automatic motion estimation on both MR-linac and MRI-simulator (Fig.3-5, respectively). Motion deconvolution was equally possible for the in-vivo data (Fig.3,4). The breathing pattern reconstructed using template-matching in the coronal plane aligns with the pattern reconstructed with the image navigator (blue box) used for validation (Fig.3). This gives confidence in the correct separation between cardiac and breathing components on MR-linac. Motion amplitude variations are attributable to the different anatomical landmark locations. In Fig. 5, the comparison between automatic template-matching with manual landmark annotations, used as independent reference, show good agreement. The close agreement between the two independent approaches shown by the MAD and RMSD values reported in the figure indicates that motion components can be quantified (semi-)automatically.Discussion
Adapting to both cardiac and respiratory motion during cardiac radioablation is an unprecedented challenge for radiotherapy. The need for tracking the cardiac target during treatment delivery necessitates the use of online MRI-guidance. While the Unity MR-linac does not currently offer any dedicated cardiac imaging hardware and is equipped with fewer receiver coils than the MRI-simulator, we were able to extract all motion components using our RTE, free-breathing, cardiac cine-MRI approach. Due to the lack of an in-vivo ground-truth, we first established the performance of our setup in a moving phantom. For the in-vivo data, we observed that our automated target tracking typically agreed with observer determined motion within 2 mm. One important limitation of this study is the reliance on a balanced-GRE sequence. Most STAR patients have ICDs, which may cause severe image artefacts, especially for a treatment target in the heart. Alternatively, other sequences (e.g., T1-GRE cine-MRI) are currently under investigation. One advantage of our proposed MRI-guided STAR workflow is that any ICD-related issues will be detected during MRI simulation, allowing for patient referral to CT-guided radiotherapy if artefacts are too prohibitive.Conclusion
This work presents the first steps towards MRI-guided STAR treatments on 1.5T Unity MR-linacs. While MR-linacs currently lack cardiac imaging hardware, we were still able to acquire RTE, free-breathing, cardiac cine-MRIs at 14 Hz without any cardiac synchronization, enabling automatic extraction of cardiac and respiratory motion. Motion estimation worked comparably well on the 1.5T MRI-simulator, potentially allowing for scar motion estimation during treatment planning. Next steps include translation to 3D motion estimation, and a phantom demonstration combining respiratory target tracking with cardiac delivery gating.Acknowledgements
MF Fast acknowledges funding by the Dutch Research Council (NWO) through project no. 17515 (BREATHE EASY). The authors are grateful to the UMC Utrecht STAR team, and Philips Healthcare for supporting this project. O. Akdag and S. Mandija contributed equally as first authors.
References
1) Phillip Cuculich, Matthew Schill, Rojano Kashani, Sasa Mutic, Adam Lang, Daniel Cooper, Mitchell Faddis, Marye Gleva, Amit Noheria, Timothy Smith, Dennis Hallahan, Yoram Rudy, Clifford G. Robinson. Noninvasive Cardiac Radiation for Ablation of Ventricular Tachycardia. N Engl J Med 2017;377:2325-36. doi: 10.1056/NEJMoa1613773
2) Raphaël Jumeau, Mahmut Ozsahin, Juerg Schwitter, Véronique Vallet, Frédéric Duclos, Michele Zeverino, Raphaël Moeckli, Etienne Pruvot, Jean Bourhis. First in human Rescue procedure for an electrical storm using robotic non-invasive cardiac radio-ablation. Radiotherapy and Oncology 128 (2018) 189–191. doi: 10.1016/j.radonc.2018.04.025.
3) Osman Akdag, Pim Borman, Jan Kok, Bram Asselen, Bas Raaymakers, Jan Lagendijk, Martin Fast. Cardiac Gating On the MR-Linac: A New Paradigm for Real-Time Adaptive Radiotherapy. AAPM 2020: 52726
Figures