4009
An MR-Tracked Metallic injection needle for Distancing Radiation-sources from Sensitive tissues: Construct and Initial testing in Swine1Medicine (Cardiology), Johns Hopkins School of Medicine, Baltimore, MD, United States, 2Radiation Oncology, Johns Hopkins School of Medicine, Baltimore, MD, United States, 3Mechanical Engineering, University of Arkansas, Fayetteville, AR, United States, 4Radiology, Brigham and Women's Hospital, Boston, MA, United States, 5Siemens Medical Solutions, Boston, MA, United States
Synopsis
A metallic actively-tracked injection needle was constructed for purposes of reducing dose to normal tissues surrounding irradiated tumors in cervical cancer and prostate cancer radiation therapy. Hydrogel is injected into tissue or anatomic cavities between the tumor and normal tissues, increasing the distance from the radiation source. The needle was tested in a gynecological phantom and in swine. It provided 1.2x1.2x1.2mm3 targeting precision @16 frames-per-second navigation, supporting rapid navigation speeds currently possible only under X-ray or Ultrasound guidance. The injected topology over time was visualized during injection, allowing creation of more uniform dose-shielding regions.
Abstract
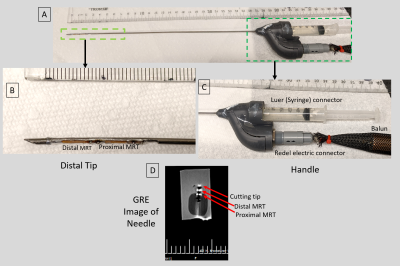
Introduction: Injection needles are used in multiple therapies (local sedation, edema reduction, chemotherapy delivery, biomarker insertion). In cancer treatment with radiation delivery or thermal ablation, injection of liquids or gels serves to distance sensitive tissues away from regions of energy delivery, reducing therapy-related complications. It is advantageous to inject under MR-guidance since the tumor and organs at risk are better visualized. Passively-tracked MR-conditional metallic needles exist, although these are only used in a restricted number of procedures, primarily due to longer navigation times relative to X-ray or Ultrasound. After using MR-tracked (MRT) metallic stylets for radiation-oncology brachytherapy (1) source deployment and elongated metallic catheters for cardiac electrophysiology ablations (2), where navigation speeds approached those under ultrasound and X-ray guidance (3), we explored use of actively-tracked metallic injection needles to displace the rectum from irradiated tissues for both cervical cancer and prostate cancer radiation therapy, based on injection of a biocompatible hydrogel (4,5). Once the needle is guided to the correct location, MR imaging can potentially monitor the topology of the hydrogel pocket during filling which allows further changes in needle location (5), thus providing a uniform (Anterior-Posterior) separation of 10-15 mm which can significantly (~80%) reduce the rectal dose (6). Efficient MRI-guided injection requires (a) MRI-conditional injection needles with large (Young’s, Torsional) elastic moduli for effective steering, and (b) sufficient SNR MRT to allow needle-tip tracking at ~1x1x1 mm3 resolution and at speeds of 12-14 frames-per-second (Ultrasound).Methods: The 1.5T actively-tracked injection needle construct: The injection needle (Figure 1) was formed from two concentric titanium tubes (0.81 mm OD, 0.1mm wall and 1.6mm OD, 0.2 mm wall, respectively) bonded to each other. The inner tube served as the liquid/gel lumen, while the space between the inner and outer tubes served as the location of the two flexible printed circuit MRT coils with embedded 63.8 MHz tuning & matching capacitors (1,2) and 46-gage micro-coaxial cables running up the shaft. To enable the MRT radio-frequency (RF) lobes to see the surrounding media, two 14-mm long groves were cut in the external titanium tube. At the distal end of the device, a 30° bevel cutting tip was created. At the proximal end of the 24 cm long needle (required for prostate cancer trans-perineal approach (4)), the micro-coax were channeled via a tuning, matching and active decoupling circuit in the handle to a quick-disconnect Redel (Lemo, Switzerland) connector. The fluid lumen was connected to a standard Luer adaptor, enabling connection of syringes. Further up the MRI front-end, signals propagated on half-wavelength coaxial cables, overlaid with 30-cm periodic resonant RF traps (Baluns), to an 8-channel preamplifier. Experiments were conducted using a dedicated MR-tracking sequence that reconstructed the needle-tip location and orientation in <0.1ms on the scanner’s reconstruction processor. For navigational display of the instantaneous needle-tip location and orientation, the tip locations were sent to a workstation equipped with a 3D Slicer MR-Tracking module (Slicer version 4.11.20200930), overlaid on MR navigational roadmaps, and displayed (1,3) on an in-room monitor. Experiments:
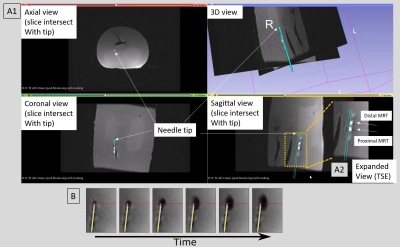
A. Phantom experiments were performed in a 70-cm bore MRI using a custom female-pelvis phantom (Figure 2). A T1-weighted Turbo-Spin-Echo (TSE) image dataset (TR\TE\Ɵ=500ms\3ms\900, ETL=6, 0.6x0.6mmx3.0mm3, 64 slice, 2min acquisition) was acquired covering the phantom using an array combining the scanner body and spine arrays along with the MRT coils, and used as a navigational roadmap. MR-Tracking (1.1x1.1x1.1mm3 resolution, Hadamard encoding, 5 phase-dithering-directions/projection, 7 averages, 16 fps) was used during navigation. To show dynamic changes in the injected volume during water injection at the desired phantom location, 2D GRE images (TR\TE\Ɵ=10ms\3ms\500, 2.0x2.0x4.5mm3, 5 slice/sec) were acquired for 20sec.
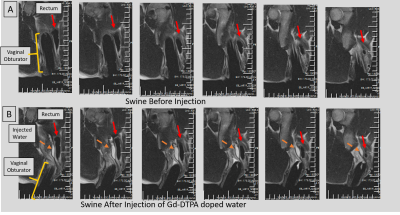
B. Swine experiment: A vaginal obturator, which spatially-anchors the brachytherapy radiation sources, was placed in the vaginal-canal. The injection-needle was used with a T2-weighted (TR\TE\Ɵ=2000ms\101ms\1800, 0.8x0.8x3mm3, ETL=12, 32 slice, 1:30min acquisition) roadmap, to actively navigate and inject Gd-DTPA-doped water into a pocket between the vaginal-canal and rectum.
Results: Navigational Precision and speed: Comparing MRI images of the beveled tip at the target location to its MR-Tracked location (Figure 2A1), the tracking precision was 1.2x1.2x1.2mm3. GRE and TSE images of the deflectable needle tip (Figure 1B, 2A2) showed hyperintense regions at the tip (in GRE-only) as well as at the MRT coils. MRT SNR supported 16 fps navigation with robust (<1mm, <5o degree deviation) visualization of the tip location and orientation. Imaging of water-filling in phantom: MR-guided injection (Figure 2B) displayed the water-filling direction and volume over time. Swine imaging of Gd-DTPA-filled pocket: MR-guided navigation, followed by T2-w monitoring (Figure 3), was used to separate the vaginal wall and rectum, visualizing the pocket topology.
Conclusions: The MR-Tracked metallic injection needle allowed rapid navigation to the desired targets, as well as accurate real-time assessment of the injected topology.
Acknowledgements
NIH R01CA237005, R01HL094610References
[1] Wang W, Dumoulin CL, Viswanathan AN, Tse ZTH, Mehrtash A, Loew W, Norton I, Tokuda J, Seethamraju RT, Kapur T, Damato AL, Cormack RA, Schmidt EJ. Real-time active MR-tracking of metallic stylets in MR-guided radiation therapy. Mag Reson Med. 2015; 73: 1803-11.
[2] Alipour A, Meyer ES, Tau S, Guttman M, Kolandaivelu A, Dumoulin C, Watkins R, Elahi H, Loew W, Schweitzer J, Olson G, Chen Y, Halperin HR, Schmidt EJ. MRI Conditional Actively Tracked Metallic Electrophysiology Catheters and Guidewires with Miniature Tethered Radio-Frequency Traps: Theory, Design and Validation. IEEE transactions on Biomedical engineering 2020;67(6),1616-1627.
[3] de Arcos J, Schmidt EJ, Wang W, Tokuda J, Vij K, Seethamraju RT, Damato AL, Dumoulin CL, Cormack RA, Viswanathan AN. Prospective Clinical Implementation of a Novel Magnetic Resonance Tracking Device for Real-Time Brachytherapy Catheter Positioning. International journal of radiation oncology, biology, physics 2017;99(3):618-626.
[4] Hatiboglu G, Pinkawa M, Vall J-P, Hadaschik B, Hohenfellner M. Application technique: placement of a prostate – rectum spacer in men undergoing prostate radiation therapy, BJUI 2012;110: E647-E652
[5] Damato AL, Kassick M, Viswanathan AN. Rectum and bladder spacing in cervical cancer brachytherapy using a novel injectable hydrogel compound (TraceIT). Brachytherapy. 2017;16(5):949-955.
[6] Marnitz S, Budach V, Weisser F, Burova E, Gebauer B, Vercellino FG, Köhler C. Rectum separation in patients with cervical cancer for treatment planning in primary chemo-radiation. Radiat Oncol. 2012;7: 109-116.
Figures