4006
Optimized passive marker device visibility for MRI-guided endovascular interventions at 3T: a pulsatile flow phantom study1Radboudumc, Nijmegen, Netherlands, 2Nano4Imaging, Düsseldorf, Germany, 3Fraunhover MEVIS, Bremen, Germany, 4Soteria Medical B.V., Arnhem, Netherlands
Synopsis
MRI-guidance for endovascular interventions is only eligible with clearly visible endovascular devices. Hence, we quantitatively and qualitatively evaluated marker visibility and artifact size of passive marker guidewires for different MRI sequence types, MRI parameters and marker concentrations at 3T MRI using a pulsatile flow phantom. Artifact size was positively correlated with TE and marker concentration and was significantly larger for bSSFP images compared to GRE images. The GRE images outperformed the bSSFP images in the quantitative image quality assessment. In conclusion, markers were adequately visible in GRE images and artifact visibility can be optimized by adjusting TE and marker concentration.
Purpose
In comparison to conventional fluoroscopy-guided endovascular interventions, MRI-guidance may profit from the associated high soft-tissue contrast, three-dimensional visualization, functional imaging and the elimination of radiation and contrast agents.1,2 MRI-visibility and guidance can be achieved by enhancing MRI-compatible vascular devices with passive paramagnetic markers, which induces susceptibility artifacts.Optimized marker visibility is crucial for successful MRI-guided endovascular interventions but from static phantom experiments it is known that marker artifact appearance is highly dependent on MRI sequence types, imaging parameters, field strength and marker characteristics.3,4 However, artifact visibility under pulsatile flow has not been investigated. Furthermore, literature regarding endovascular device visibility focusing on endovascular interventions at higher field strengths (>1.5T) is scarce. Hence, this study compared artifact sizes and visibility of customized existing commercial passive marker guidewires for different MRI parameters and marker concentrations using 3T MR imaging in an iliac artery-mimicking pulsatile flow phantom.
Methods
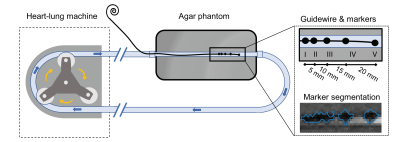
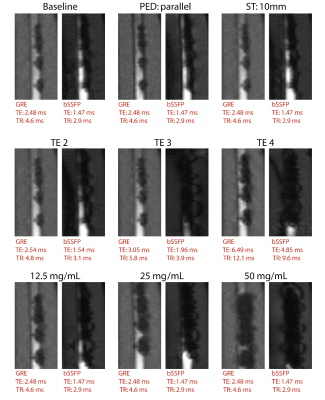
A vascular flow phantom was designed by emerging an 8-mm tube, comparable to the diameter of the iliac artery, in a soft tissue-mimicking agar solution (Sigma-Aldrich, St. Louis, Missouri, US) and connecting the tube to a heart-lung machine (HL 20, Getinge, Gothenburg, Sweden) (Figure 1). To mimic human blood flow, a blood-mimicking fluid (3% glycerol in water doped with 0.15 ml/L Dotarem)5 was pumped through the tubing with a pulsatile flow of 0.4 L/min at 60 beats per minute.Four guidewires (EmeryGlide®, Nano4Imaging GmbH, Düsseldorf, Germany) were modified with varying iron(II,III)oxide (Fe3O4) nanoparticle concentration markers (6.25, 12.5, 25, and 50 mg/mL) and imaged using two baseline MRI sequences commonly used to guide interventional endovascular procedures; (i) a 2D spoiled gradient recalled echo (GRE) sequence (echo time (TE)/ repetition time (TR): 2.48/4.6 ms, framerate: 3.3 frames per second (fps), matrix size 144x144, flip angle (FA): 12ᴼ, pixel size: 1.74x1.74 mm, slice orientation: sagittal, phase encoding direction (PED): perpendicular to B0, slice thickness (ST): 5 mm) with GRAPPA (2-fold, 24 reference lines) and partial Fourier (6/8); (ii) a 2D balanced steady-state free precession (bSSFP) sequence with equal parameters, except for TE/TR (1.47/2.9 ms), FA (39ᴼ) and framerate (5 fps). Furthermore, echo time (GRE: 2.48, 2.54, 3.05, and 6.49 ms, bSSFP: 1.47, 1.54, 1.96, and 4.85 ms), slice thickness (5 and 10 mm) and phase encoding direction (perpendicular and parallel) were varied for both sequences. Lastly, reference images of the phantom without guidewire were acquired.
Automatic segmentation of the image artifact was performed by subtracting the marker images from the paired reference images and applying a standardized threshold (mean + 3 times the standard deviation of the agar signal intensity) (Figure 1). Marker artifact width and contrast-to-noise (CNR) ratios between blood and artifact (blood-artifact CNR) and between agar and artifact (agar-artifact CNR) were compared between different parameters and sequence types using the Wilcoxon signed-rank test. Furthermore, image quality assessment was performed by two MRI-interventionalists, evaluating marker visibility, overall image quality and usefulness for guidance of vascular interventions using a 5-point Likert scale (unacceptable (1), poor (2), acceptable (3), good (4), excellent (5)).
Results
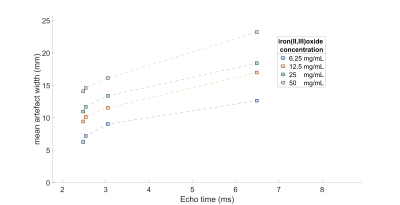
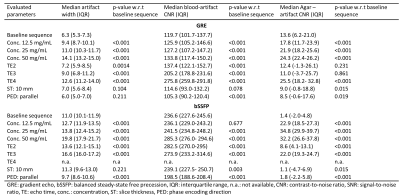
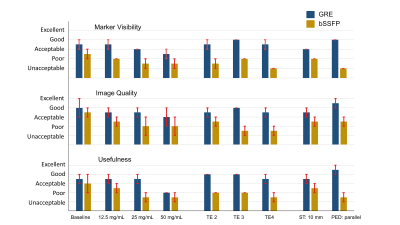
Median artifact width was significantly larger in bSSFP compared to GRE images (13.6 ± 3.3 vs. 9.1 ± 2.8 mm; p<0.001) and showed a positive relation with TE and marker concentration (Figure 3, Table 1). Switching PED and doubling ST had limited effect on artifact width (<1.5mm). The CNR between artifact and blood was significantly lower for GRE images compared to bSSFP images (149.4 +/- 55.4 vs. 249.2 +/- 29.5; p < 0.001). The GRE images outperformed the bSSFP images in the image quality assessment and scored acceptable to good (Figure 4).Discussion
Our results showed that the artifact size of the passive markers showed a positive relation with TE and the iron oxide concentration. Comparing GRE with the bSSFP showed significantly smaller artifact sizes and better image quality for the GRE sequence. Those findings are in concordance with results from static phantom experiments, which showed increasing artifact widths for larger TE’s and higher marker concentrations.6 The inferior image quality of the bSSFP images can be explained by the higher sensitivity for field inhomogeneities.7Based on our results, we recommend using the GRE sequence for MRI-guidance of endovascular interventions at 3T. Furthermore, marker concentrations up to 12.5 mg/mL resulted in artifact sizes < 10 mm in GRE images, appearing clinically acceptable. The realistic pulsatile flow resulted in variations in CNR and artifact width in consecutive images, which can impede automatization of marker detection.
Strength of this study is the use of generally accepted MRI sequences for MRI-guided endovascular interventions in a pulsatile flow phantom. Also, quantitative and qualitative analysis on artifact appearance were combined to investigate objective changes as well as applicability and usefulness according to expert evaluation. Although our results are promising and may provide directions in optimizing passive marker visibility, in-vivo evaluation is required to justify clinical implementation.
Conclusion
Passive marker enhanced endovascular devices were adequately displayed at 3T MRI in a pulsatile flow phantom in GRE and poorly in bSSFP images. By varying TE and iron(II,II)oxide concentration, a large range of artifact sizes can be achieved. Concentrations up to 12.5 mg/mL resulted in clinically acceptable artifact sizes, especially for peripheral interventions.Acknowledgements
This research was financially supported by the Eurostars - Eureka program (SPECTRE E! 11263). We thank Nano4Imaging for their contribution in the guidewire design and the supply of customized EmeryGlide® guidewires.References
1. Heidt T, Reiss S, Krafft AJ, et al. Real-time magnetic resonance imaging – guided coronary intervention in a porcine model. Sci Rep. 2019;9(1):1-10. doi:10.1038/s41598-019-45154-7
2. Wendt D, Thielmann M, Melzer A, et al. The past, present and future of minimally invasive therapy in endovascular interventions: A review and speculative outlook. Minim Invasive Ther Allied Technol. 2013;22(4):242-253. doi:10.3109/13645706.2013.822396
3. Settecase F, Martin AJ, Lillaney P, Losey A, Hetts SW. Magnetic Resonance-Guided Passive Catheter Tracking for Endovascular Therapy. Magn Reson Imaging Clin N Am. 2015;23(4):591-605. doi:10.1016/j.mric.2015.05.003
4. Ariens M, Bruhn R, Liess C, et al. The depiction of labeled guidewires in a phantom in an interventional MRI setting. Biomed Tech. 2013;58(SUPPL.1). doi:10.1515/bmt-2013-4295
5. Storey P, Atanasova IP, Lim RP, et al. Tailoring the flow sensitivity of fast spin-echo sequences for noncontrast peripheral MR angiography. Magn Reson Med. 2010;64(4):1098-1108. doi:10.1002/mrm.22510
6. Bos C, Viergever MA, Bakker CJG. On the artifact of a subvoxel susceptibility deviation in spoiled gradient-echo imaging. Magn Reson Med. 2003;50(2):400-404. doi:10.1002/mrm.10505
7. Li X, Perotti LE, Martinez JA, Duarte-Vogel SM, Ennis DB, Wu HH. Real-time 3T MRI-guided cardiovascular catheterization in a porcine model using a glass-fiber epoxy-based guidewire. PLoS One. 2020;15(2):e0229711. doi:10.1371/journal.pone.0229711
Figures