3999
Quantitative susceptibility mapping in the basal ganglia of systemic lupus erythematosus patients with neuropsychiatric complaints1Leiden University Medical Center, Leiden, Netherlands, 2Zuyderland Medical Center, Heerlen, Netherlands, 3Otto-von-Guericke University, Magdeburg, Germany
Synopsis
We explored the link between iron accumulation and neuroinflammation in basal ganglia in SLE using quantitative susceptibility mapping. We hypothesized that SLE patients, and in particular NPSLE patients would have increased numbers of activated microglia co-localizing with iron, which in turn would be reflected in increased local susceptibility. Based on QSM results in patients, as well as on iron staining of post-mortem SLE brain tissue, our results suggest that neuroinflammation in NPSLE is not necessarily associated with iron accumulation, and that the inflammatory pathomechanism in SLE may differ from the one observed in neurodegenerative diseases and in multiple sclerosis.
Introduction
Systemic lupus erythematosus (SLE) is an auto-immune disease characterized by multi-organ involvement. SLE is the prototype of a systemic disease that can also present with a variety of neurological and psychiatric complaints, termed neuropsychiatric SLE (NPSLE)1. The precise etiology of NPSLE remains unknown. Currently, the two main underlying pathogenic mechanisms of NPSLE are thought to be ischemia and inflammation2. Although there is in-vivo and post-mortem evidence of neuroinflammation in NPSLE3-4, no direct neuroimaging technique has so far contributed to explain the exact mechanism that causes neuroinflammation in NPSLE, or gave direct evidence of neuroinflammation in-vivo. Quantitative MRI techniques such as magnetization transfer imaging (MTI) and diffusion tensor imaging (DTI) have proven to be useful in identifying microstructural alterations that can be attributed to the effects of neuroinflammation in NPSLE5-6. Both techniques, however, only provide indirect evidence for inflammation and may also reflect other underlying pathophysiological processes unrelated to inflammation.Increasing amount of evidence from several diseases suggests that neuroinflammation, characterized by microglia activation and proinflammatory cytokines, is highly correlated with brain iron accumulation. Thus, quantitative susceptibility mapping (QSM) could be a potential neuroimaging marker for neuroinflammation in vivo. QSM has already been applied in several neurodegenerative diseases, but also in (neuro)inflammatory diseases such as multiple sclerosis7 and SLE8.
We used QSM to investigate the potential role of iron accumulation in the brain of NPSLE patients. We assessed susceptibility values of the basal ganglia and compared the susceptibility values of the healthy controls to those of the patient group as a whole, as well as to subgroups of patients, stratified according to the origin of their neuropsychiatric complaints (related or unrelated to SLE). In addition, we investigated the correlation with clinical variables such as SLE disease activity and SLE damage. Finally, we further explain our in-vivo findings with histological analyses of post-mortem brain tissue of three SLE patients stained for iron and activated glia.
Methods
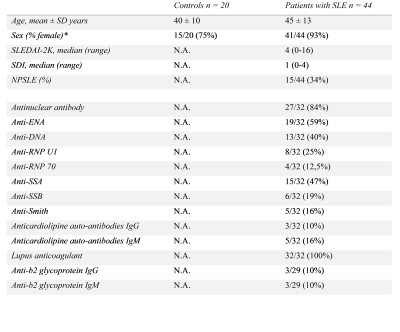
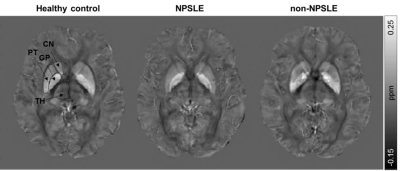
In total 44 SLE patients and 20 age-matched healthy controls were included in this study. All patients had neuropsychiatric complaints, 29 of them were classified as non-NPSLE and 15 as NPSLE (seven as inflammatory NPSLE and eight as ischemic NPSLE) (Fig.1). Single-echo T2*-weighted images (0.78x0.78x0.78mm, TR/TE=45/31ms, flip angle 13°) were acquired on a 3T Philips Achieva equipped with an 8-channel receive coil. The QSM maps were reconstructed using STI Suite9. A Laplacian-based method was used for phase unwrapping, followed by background field removal using VSHARP10 and dipole inversion using iLSQR10. Mean susceptibility values of the thalamus, caudate nucleus, putamen, and globus pallidus were calculated for each subject from the registered QSM maps in MNI space (Fig.2). Formalin-fixed paraffin-embedded post-mortem brain tissue samples of three SLE patients, including the putamen and globus pallidus, were stained for iron, microglia and astrocytes. Statistical analyses were performed with SPSS.Results
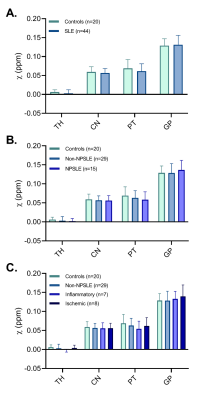
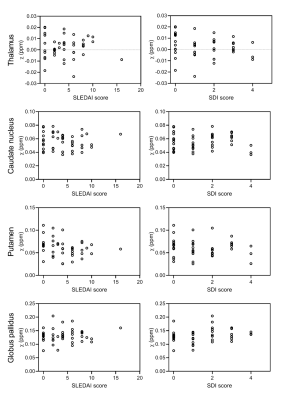
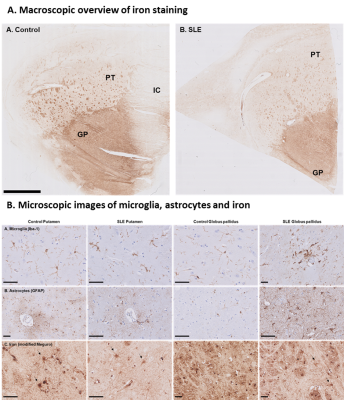
Susceptibility values of SLE patients and age-matched controls showed that iron levels in the basal ganglia were not changed due to the disease (Fig.3). No subgroup of SLE showed higher susceptibility values. Although all analyses were done in MNI152 space, we performed a volumetric comparison of the individual regions of interest to exclude confounding effects due to between-subject differences in volume. Volumes of the thalamus were significantly larger in SLE patients compared to controls (p=0.012 for T1 volumes, p=0.014 for T1 in MNI space volumes). However, comparison of susceptibility values of the thalamus corrected for volume did not change the results. To rule out more focal differences in QSM maps between SLE patients and controls, we performed a voxel based QSM analysis on the thalamus, caudate nucleus, putamen, and globus pallidus. Also voxel-based comparisons did not show any significant differences between SLE patients and controls. No correlation was found with disease activity or damage due to SLE (Fig.4).Macroscopic examination of the iron staining showed higher staining intensity in the globus pallidus compared to the putamen, closely resembling the in vivo susceptibility findings (Fig.5). The amount of iron in cells morphologically resembling microglia and astrocytes was not visibly different in SLE brains compared to control brains (Fig.5).
Discussion & Conclusion
Our main aim in this study was to explore the potential link between iron accumulation and neuroinflammation in SLE, following several findings that point to such a link in neurodegenerative diseases such as Alzheimer’s disease and Huntington’s disease, and mainly in multiple sclerosis, an autoimmune disease that shares some common aspects with SLE. We hypothesized that similar to the diseases previously mentioned, SLE patients and in particular NPSLE patients would have increased numbers of activated microglia co-localizing with iron, which in turn would be reflected in increased local susceptibility.This study did not find susceptibility changes in the basal ganglia of SLE patients, and in that respect NPSLE might behave differently than other neuroinflammatory and neurodegenerative diseases such as multiple sclerosis. However, as conclusive evidence is lacking, this should be further investigated and may offer opportunities for differential diagnosis and further insights into the diverging pathomechanisms of neuroinflammatory diseases. More data from both post-mortem (NP)SLE brains as well as more in-vivo data from independent sources such as iron concentrations in cerebrospinal fluid are needed to obtain a better picture of iron involvement in (NP)SLE.
Acknowledgements
The authors would like to thank R.C. Monahan for her help in collecting the clinical data. This work was supported by a grant from ZONMW program Innovative Medical Devices Initiative, project Imaging Dementia: Brain Matters (104003005).References
1. Jeltsch-David H, Muller S. Neuropsychiatric systemic lupus erythematosus: Pathogenesis and biomarkers. Nat Rev Neurol. 2014.
2. Zirkzee EJ, Steup-Beekman GM, van der Mast RC, Bollen EL, van der Wee NJ, Baptist E, et al. Prospective study of clinical phenotypes in neuropsychiatric systemic lupus erythematosus; multidisciplinary approach to diagnosis and therapy. J Rheumatol. 2012.
3. Cohen D, Rijnink EC, Nabuurs RJ, Steup-Beekman GM, Versluis MJ, Emmer BJ, et al. Brain histopathology in patients with systemic lupus erythematosus: Identification of lesions associated with clinical neuropsychiatric lupus syndromes and the role of complement. Rheumatology (Oxford). 2017. 4. Curiel R, Akin EA, Beaulieu G, DePalma L, Hashefi M. Pet/ct imaging in systemic lupus erythematosus. Ann N Y Acad Sci. 2011.
5. Ercan E, Ingo C, Tritanon O, Magro-Checa C, Smith A, Smith S, et al. A multimodal mri approach to identify and characterize microstructural brain changes in neuropsychiatric systemic lupus erythematosus. Neuroimage Clin. 2015.
6. Nystedt J, Nilsson M, Jonsen A, Nilsson P, Bengtsson A, Lilja A, et al. Altered white matter microstructure in lupus patients: A diffusion tensor imaging study. Arthritis Res Ther. 2018.
7. Haider L, Simeonidou C, Steinberger G, Hametner S, Grigoriadis N, Deretzi G, et al. Multiple sclerosis deep grey matter: The relation between demyelination, neurodegeneration, inflammation and iron. J Neurol Neurosurg Psychiatry. 2014.
8. Ogasawara A, Kakeda S, Watanabe K, Ide S, Ueda I, Murakami Y, et al. Quantitative susceptibility mapping in patients with systemic lupus erythematosus: Detection of abnormalities in normal-appearing basal ganglia. Eur Radiol. 2016.
9. Li W, Avram AV, Wu B, Xiao X, Liu C. Integrated laplacian-based phase unwrapping and background phase removal for quantitative susceptibility mapping. NMR Biomed. 2014.
10. Li W, Wu B, Liu C. Quantitative susceptibility mapping of human brain reflects spatial variation in tissue composition. Neuroimage. 2011.
Figures