3990
Quantitative susceptibility mapping in the infant brain diagnosed with congenital heart disease1Division of Diagnostic Imaging and Radiology, Children's National Hospital, Washington, DC, United States, 2Division of Fetal and Transitional Medicine, Children's National Hospital, Washington, DC, United States, 3Department of Pediatrics, George Washington University, Washington, DC, United States, 4Department of Radiology, George Washington University, Washington, DC, United States, 5Division of Cardiology, Children’s National Hospital, Washington, DC, United States
Synopsis
Quantitative susceptibility mapping may be used to assess brain development in infants based on its ability to evaluate iron and myelin contents. We measured magnetic susceptibilities in healthy infants and those diagnosed with congenital heart disease (CHD) in the early postnatal period and investigated the associations with neurodevelopmental outcomes. We report significantly lower magnetic susceptibilities in white matter and temporal lobe of the CHD cohort and significant associations between magnetic susceptibility of the temporal lobe and Bayley-III language scores at 18 months. Our findings may suggest disturbed iron deposition in the temporal lobe can lead to delays in language development.
INTRODUCTION
The early postnatal period is a crucial phase of rapid and complex brain development. Quantitative susceptibility mapping (QSM) is an effective method for evaluating dynamic changes of iron and myelin contents in the brain, but assessment of brain development using QSM has not previously been well established. In this study we measured magnetic susceptibilities in the brain of healthy infants and those diagnosed with congenital heart disease (CHD) in the early postnatal period and investigated their associations with neurodevelopmental outcomes.METHODS
In this prospective study, enrolled healthy full-term infants underwent one term MRI exam and infants diagnosed with CHD underwent pre-operative and post-operative MRI exams. All MRI scans were performed on a GE MR 750 3T scanner using an 8-channel head coil. MR data for QSM were acquired using a 3D multi-echo gradient echo sequence with imaging parameters of TR = 55 ms, TE = 5.8, 12.2, 18.6, 25.0, 31.4, 37.8, and 44.2 ms, bandwidth = ±50 kHz, flip-angle = 20°, field-of-view = 160 x 128 x 120 mm3, spatial revolution = 0.38 x 0.4 x 2 mm3, and scan time of 5 min. QSM reconstruction was performed using morphology enabled dipole inversion method.1,2 The reconstructed susceptibility maps were registered to T2-weighted anatomical images, which were segmented using Draw-EM.3 Our regions-of-interest (ROIs) included cortical gray matter (CGM), white matter (WM), deep gray matter (DGM), cerebellum, and brainstem. Within the DGM, caudate nucleus (CN), lentiform nucleus (LN), and thalamus were further segmented. CGM and WM were also divided into the four lobes of the brain, yielding frontal GM (FGM), parietal GM (PGM), temporal GM (TGM), occipital GM (OGM), frontal WM (FWM), parietal WM (PWM), temporal WM (TWM), and occipital WM (OWM). Neurodevelopmental outcome at 18 months was assessed in our cohorts using the Bayley Scales of Infant and Toddler Development, Third Edition (Bayley-III).4 This study was approved by the local institutional review board and written informed consent was obtained from the parents of each infant.RESULTS
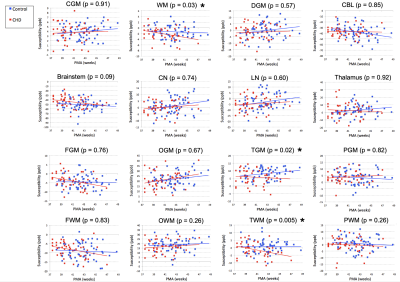
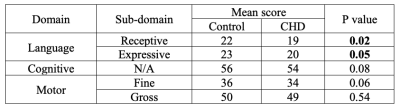
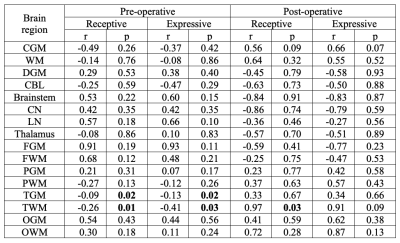
A total of 60 healthy infants (postmenstrual age [PMA] = 42.6 ± 2.4, ranging 38 1/7 – 48 5/7 weeks) and 30 infants with CHD were studied. Of the 30 infants with CHD, 24 infants underwent pre-operative MRI (PMA = 39.1 ± 1.3, ranging 37 3/7 – 43 1/7 weeks) and 21 underwent post-operative (PMA = 42.6 ± 1.8, ranging 39 3/7 – 47 weeks) MRI. Among our ROIs, there were significant differences in mean susceptibility between control and CHD infants in WM, temporal GM, and temporal WM when controlling for PMA and accounting for repeated (i.e., pre- and post-operative) measures of the same individual (Figure 1). Follow-up neurodevelopmental examinations were performed at 18 months of age in 35 control infants and 9 CHD infants. In these infants, Bayley raw scores were significantly lower in CHD infants compared to controls in the receptive and expressive language domains (Table 1). Associations between magnetic susceptibilities and Bayley language scores were evaluated in CHD infants who underwent pre-operative (n=8) and post-operative (n=4) MRI (Table 2). Bayley language scores were significantly associated with susceptibility measurements in the temporal lobes.DISCUSSION
We report significantly lower magnetic susceptibilities in WM and temporal lobe of the infants diagnosed with CHD. Since both GM and WM of the temporal lobe showed lower susceptibility, the underlying source of lower susceptibility in the temporal lobe of the CHD cohort may be lower iron content. And lower susceptibility in temporal WM may contribute substantially to lower susceptibility in total WM. We postulate that associations between magnetic susceptibility and Bayley language scores suggest disturbed iron deposition in the temporal lobe in the early postnatal period impairs language development. Follow-up studies are warranted to validate the relationship between magnetic susceptibility and iron content in the infant brain.Acknowledgements
R01HL116585, R01HD100012, U54HD090257, R01HD099393References
1. Liu J, Liu T, de Rochefort L, Ledoux J, Khalidov I, Chen W, Tsiouris AJ, Wisnieff C, Spincemaille P, Prince MR, Wang Y. Morphology enabled dipole inversion for quantitative susceptibility mapping using structural consistency between the magnitude image and the susceptibility map. Neuroimage. 2012;59:2560-2568.
2. Liu T, Liu J, de Rochefort L, Spincemaille P, Khalidov I, Ledoux JR, Wang Y. Morphology enabled dipole inversion (MEDI) from a single-angle acquisition: Comparison with COSMOS in human brain imaging. Magn Reson Med. 2011;66:777-783.
3. Makropoulos A, Gousias IS, Ledig C, Aljabar P, Serag A, Hajnal JV, Edwards AD, Counsell SJ, Rueckert D, Automatic whole brain MRI segmentation of the developing neonatal brain. IEEE Trans Med Imaging. 2014;33,1818-1831.
4. Bayley N. Bayley scales of infant and toddler development. 3rd ed. San Antonio, TX: Pearson Education; 2006.
Figures