3967
Rotation-Free Mapping of Magnetic Tissue Properties in White Matter1Department of Clinical Medicine, Center for Functionally Integrative Neuroscience, Aarhus University, Aarhus, Denmark, 2Medical Physics, Department of Radiology, Faculty of Medicine, University of Freiburg, Freiburg, Germany, 3Champalimaud Neuroscience Programme, Champalimaud Centre for the Unknown, Lisbon, Portugal, 4Department of Physics and Astronomy, Aarhus University, Aarhus, Denmark
Synopsis
Mapping tissue magnetic properties may be a useful tool for understanding basic disease mechanism in neurodegenerative diseases. However, magnetic properties depend on the arrangement of magnetic susceptibility on all scales, including the microstructure, and typically require impractical sample rotations for robust estimation. By modeling white matter fibers as a system of cylinders, here we show that the first cumulant $$$\langle\Omega\rangle$$$ of the FID signal can be obtained without the need for sample rotation, by utilizing the fiber orientation distribution function estimated by diffusion imaging.
Introduction
Features such as brain-calcification1 demyelination2 and iron-dyshomeostasis3 are associated with various neurodegenerative diseases, yet their noninvasive characterisation is a formidable challenge. MRI-driven mapping of magnetic tissue properties, including the local Larmor frequency, is promising for providing insights into such features, but both susceptibility and microstructure play important roles in the signal formation4,5,6,7, which hampers accurate measurements without impractical sample rotations. The aim of the study is to bypass sample rotations by combining diffusion MRI and susceptibility-based MRI, which enables utilising information from the fiber orientation distribution function (fODF) as determined by diffusion imaging9. This, in turn, enables the estimation of the Larmor frequency and thereby a new means for characterising magnetic properties without sample rotation.Theory
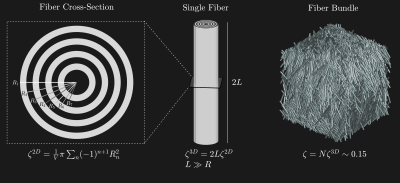
We model white matter as a medium of long hollow impermeable cylindrical inclusions. The inclusions are made of concentric shells, representing myelin, with an isotropic susceptibility $$$\chi$$$ relative to the surrounding MRI-visible fluid (figure 1), as a first approximation. The surroundings correspond to intra-axonal, extra-axonal and interstitial bi-layers. The inclusions occupy $$$\zeta{V}$$$ of the total volume $$$V,$$$ and are placed according to the indicator function $$$\nu(\mathbf{r})$$$. Placed in a static field $$$(B_0)_\alpha=B_0{{n}}_{\alpha}^{{B}},$$$ a shift in the local Larmor frequency $$\Omega(r)=\gamma B_{0}\chi{n}_{\alpha}^{B}{n}_{\beta}^{B}\int_{V}d\mathbf{r}^{\prime}\mathcal{Y}_{\alpha\beta}\left(\mathbf{r}^{\prime}-\mathbf{r}\right)\nu\left(\mathbf{r}^{\prime}\right)\qquad\qquad(1)$$ is induced in the fluid, where $$$\mathcal{Y}_{\alpha\beta}$$$ is the Lorentz-corrected elementary dipole-field8. The normalized FID signal $$$S_k(t)$$$ from the MRI-visible fluid within an Isotropic voxel $$$V_k\in{V}$$$ is given by the ensemble average $$$\langle\cdot\rangle$$$ $$S_{k}(t)=e^{-R_{2}^{mol}t}\left\langle{e}^{-i\varphi_{k}(t)}\right\rangle,\qquad\varphi_{k}(t)=\int_{0}^{t}dt^{\prime} \Omega_{\mathrm{k}}\left(\mathbf{r}\left(t^{\prime}\right)\right).\qquad\qquad(2)$$ Here, $$$R_{2}^{mol}$$$ accounts for the irreversible molecular relaxation10. The first cumulant $$$\langle\Omega\rangle_k$$$ can be found from the signal derivative $$-Im\left\{\left.\frac{d}{dt}S_{k}(t)\right|_{t\rightarrow{0}}\right\}=\langle\Omega\rangle_{k},\qquad\qquad(3)$$ and $$$\langle\Omega\rangle_k$$$ may be obtained as $$\langle\Omega\rangle_{k}=\gamma{B}_{0}\chi{n}_{\alpha}^{B}{n}_{\beta}^{B}\frac{1}{\left(1-\zeta_{k}\right)V_{k}}\int_{V_{k}}d\boldsymbol{r}(1-v(\boldsymbol{r}))\int_{V}d\boldsymbol{r}^{\prime}\mathcal{Y}_{\alpha\beta}\left(\boldsymbol{r}^{\prime}-\boldsymbol{r}\right)v\left(\boldsymbol{r}^{\prime}\right).\qquad\qquad(4)$$ $$$\langle\Omega\rangle_k$$$ is described by the average static field within $$$V_k$$$, averaged over both intra-axonal, extra-axonal and interstitial bi-layers. Within the mesoscopic cavity approach4,5,6,8, $$$\langle\Omega\rangle_k$$$ is divided into mesoscopic and macroscopic contributions, $$$\langle\Omega\rangle_k=\langle\Omega\rangle^{Meso}_k+\langle\Omega\rangle^{Macro}_k$$$, with $$\begin{array}{l}\langle\Omega\rangle_{k}^{Meso}=\gamma{B}_{0}\chi{n}_{\alpha}^{B}{n}_{\beta}^{B}\frac{1}{\left(1-\zeta_{k}\right)V_{k}}\int_{V_{k}}d\boldsymbol{r}(1-\nu(\boldsymbol{r}))\int_{\boldsymbol{r}^{\prime}\in\mathcal{M}(\boldsymbol{r})}d\boldsymbol{r}^{\prime}\mathcal{Y}_{\alpha\beta}\left(\boldsymbol{r}^{\prime}-\boldsymbol{r}\right)\nu\left(\boldsymbol{r}^{\prime}\right)\\\langle\Omega\rangle_{k}^{Macro}=\gamma{B}_{0}\chi{n}_{\alpha}^{B}{n}_{\beta}^{B}\frac{1}{\left(1-\zeta_{k}\right)V_{k}}\int_{V_{k}}d\boldsymbol{r}(1-\nu(\boldsymbol{r}))\int_{\boldsymbol{r}^{\prime}\notin\mathcal{M}(\boldsymbol{r})}d\boldsymbol{r}^{\prime}\mathcal{Y}_{\alpha\beta}\left(\boldsymbol{r}^{\prime}-\boldsymbol{r}\right)\nu\left(\boldsymbol{r}^{\prime}\right)\end{array},\qquad\qquad(5)$$ where $$$\mathcal{M}(\boldsymbol{r})$$$ denote the mesoscopic region surrounding $$$\boldsymbol{r}$$$.Macroscopic Contribution $$$\langle\Omega\rangle^{Macro}_k$$$
$$$\langle\Omega\rangle^{Macro}_k$$$ was previously studied11 and found to be $$\langle\Omega\rangle_{k}^{Macro}\approx\gamma{B}_{0}{n}_{\alpha}^{B}{n}_{\beta}^{B}\sum_{j}\mathcal{Y}_{\alpha\beta,k,j}^{Macro}\chi_{j}^{B},\qquad\qquad(6)$$ where $$$\mathcal{Y}_{\alpha\beta,k,j}^{Macro}$$$ describes the macroscopic dipole coupling between two rectangular voxels of volume $$$V_k$$$ and $$$V_j:$$$ $$\mathcal{Y}_{\alpha\beta,k,j}^{{Macro}}\equiv\frac{1}{V_{k}}\int_{V_{k}}d\boldsymbol{r}\int_{V_{j}}d\boldsymbol{r}^{\prime}\mathcal{Y}_{\alpha\beta}\left(\boldsymbol{r}^{\prime}-\boldsymbol{r}\right)-\frac{1}{V_{k}}\int_{V_{k}}d\boldsymbol{r}\int_{\boldsymbol{r}^{\prime}\in\mathcal{M}(\boldsymbol{r})}d\boldsymbol{r}^{\prime}\mathcal{Y}_{\alpha\beta}\left(\boldsymbol{r}^{\prime}-\boldsymbol{r}\right)\mathbb{I}_{\left\{\boldsymbol{r}^{\prime}\in{V}_{j}\right\}}.$$ $$$\mathbb{I}_{\left\{\boldsymbol{r}^{\prime}\in{V}_{j}\right\}}$$$ is the indicator function stemming from $$$\mathcal{M}$$$ potentially overlapping multiple $$$V_j$$$. The bulk magnetic susceptibility is $$\chi_k^B=\zeta_k\chi.\qquad\qquad(8)$$
Mesoscopic Contribution $$$\langle\Omega\rangle^{Meso}_k$$$ for fiber bundles
Consider $$$\langle\Omega\rangle^{Meso}_k$$$ for a fiber bundle as seen in figure 1. $$$\mathcal{M}$$$ is chosen to be as $$$V_k,$$$ making the fiber bundle larger than the voxel dimensions. In the low volume-fraction limit, $$\langle\Omega\rangle_{k}^{Meso}=\gamma{B}_{0}\mathcal{Y}_{\alpha\beta,k}^{Meso}\chi_{k}^{B}{n}_{\alpha}^{B}n_{\beta}^{B}\qquad\qquad(9)$$ where $$$\mathcal{Y}_{\alpha\beta,k}^{Meso}$$$ depends on the fODF scatter matrix $$$T_{\alpha\beta}=\overline{n_{\alpha}n_{\beta}}$$$12 (average over fODF) $$\mathcal{Y}_{\alpha\beta,k}^{Meso}\approx\frac{1}{2}\left(T_{\alpha\beta,k}-\frac{1}{3}\delta_{\alpha\beta}\right).\qquad\qquad(10)$$ The scatter matrix $$$T$$$ can be expressed in terms of the Laplace expansion coefficients of the fODF $$$p_{2m}$$$, such that the mesoscopic contribution becomes $$\langle\Omega\rangle_{k}^{Meso}\approx\frac{1}{3}\gamma{B}_{0}{n}_{\alpha}^{B}{n}_{\beta}^{B} \chi_{k}^{B}\sum_{m}p_{2m,k}Y_{\alpha\beta}^{2m},\qquad\qquad(11)$$ where $$$Y_{\alpha\beta}^{2m}$$$ define the irreducible rank-2 symmetric trace-free (STF) tensor representation of SO(3)13.The total average Larmor frequency becomes $$\langle\Omega\rangle_{k}=\gamma{B}_{0}{n}_{\alpha}^{B}{n}_{\beta}^{B}\left(\frac{1}{3}\chi_{k}^{B}\sum_{m}p_{2m,k}Y_{\alpha\beta}^{2m}+\sum_{j}\mathcal{Y}_{\alpha\beta,k,j}^{Macro}\chi_{j}^{B}\right).\qquad\qquad(12)$$
Methods
Data acquisitionMulti-Gradient-Echo (MGE) and diffusion-data of an ex-vivo mouse brain were acquired at Champalimaud Centre of the Unknown, Lisbon, Portugal, on a 16.4T Bruker Ascend Aeon. MGE phase was unwrapped and background-field-removed14,15. $$$ \langle\Omega\rangle_k$$$ was estimated as the linear term in a polynomial fit of the phase for low $$$t$$$. $$$p_{lm}$$$ were estimated using CSD16, and $$$\chi^B$$$ was fitted to eqn. 13 using the LSMR algorithm17.
Computer Simulations
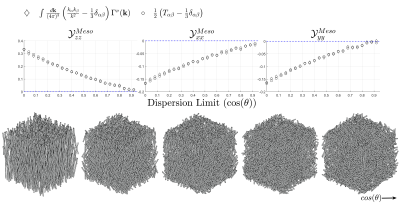
Simulations to test model assumptions were performed in Matlab. Cylinders were packed with various levels of dispersion (figure 4). Volume fractions around 15% were reached for solid fibers. Fibers were then hollowed after packing. $$$\mathcal{Y}_{\alpha\beta}^{Meso}$$$ was calculated numerically6,7 and compared with eqn. 11.
Results
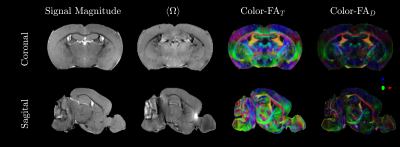
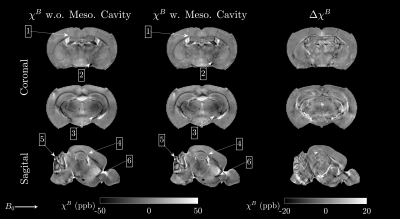
MRI dataFigure 2 gives an overview of the MGE signal magnitude, $$$\langle\Omega\rangle_k$$$ and color-coded FA of $$$T_{\alpha\beta}$$$ and diffusion tensor $$$D_{\alpha\beta}$$$. As expected,$$$T_{\alpha\beta}$$$ produced sharper colormaps than $$$D_{\alpha\beta}$$$. Figure 3 shows the susceptibility map $$$\chi^B$$$ from fitting eqn.13, with and without including $$$\langle\Omega\rangle^{Meso}_k$$$, along with the difference $$$\Delta\chi^B$$$.
Simulation
Validation of the macroscopic model assumptions for $$$\mathcal{Y}_{\alpha\beta}^{Macro}$$$ was previously presented11. Figure 4 shows the diagonal of $$$\mathcal{Y}_{\alpha\beta}^{Meso}$$$ computed numerically, and from eqn. 11.
Discussion
It is clear from figure 4 that approximating $$$\mathcal{Y}_{\alpha\beta}^{Meso}$$$ based on the fODF is in excellent agreement with explicit numerical calculation. When fiber bundles are randomly oriented, $$$\langle\Omega\rangle^{Meso}_k$$$ is zero. As dispersion decreases, $$$\langle\Omega\rangle^{Meso}_k$$$ increases becoming maximal in the parallel limit.It is also evident from the susceptibility maps in figure 3 that $$$\langle\Omega\rangle^{Meso}_k$$$ introduces noticeable changes in $$$\chi^B$$$. For example, at the isthmus of corpus callosum, the medial region is almost invisible without the mesoscopic contribution (as can be seen at point 1 and 5 in figure 3). Neglecting the mesoscopic contribution is analogous to classical QSM11.
The model can also be extended to cylinders with a radially symmetric susceptibility tensor18. However, such a model would require sample rotations. Other limitations potentially affecting the accuracy of the model include the simple voxel shapes, and assumption of cylinders19. In addition, other types of inclusions could be needed to fully characterize the shift in Larmor frequency.
Conclusion
The first cumulant of the FID signal is considered as a probe of magnetic susceptibility in white matter. As a first step towards incorporation the fODF in order to bypass sample rotations, the local susceptibility is treated as a scalar. By fitting the model to MRI data from an ex-vivo mouse brain, a higher apparent resolution was achieved in susceptibility maps.Acknowledgements
This study is funded by the Independent Research Fund (grant 8020-00158B), Denmark, and by Helga & Peter Korning's Foundation. The authors would like to thank PhD student Jonas Lynge Olesen for many fruitful discussions.References
1) Grech, R., S. Grech, and A. Mizzi. "Intracranial calcifications: a pictorial review." The neuroradiology journal 25.4 (2012): 427-451.
2) Love, Seth. "Demyelinating diseases." Journal of clinical pathology 59.11 (2006): 1151-1159.
3) Ndayisaba, Alain, Christine Kaindlstorfer, and Gregor K. Wenning. "Iron in neurodegeneration–cause or consequence?." Frontiers in neuroscience 13 (2019): 180.
4) Kiselev, Valerij G. "Larmor frequency in heterogeneous media." Journal of Magnetic Resonance 299 (2019): 168-175.
5) Ruh, Alexander, Harald Scherer, and Valerij G. Kiselev. "The larmor frequency shift in magnetically heterogeneous media depends on their mesoscopic structure." Magnetic Resonance in Medicine 79.2 (2018): 1101-1110.
6) Ruh, Alexander, and Valerij G. Kiselev. "Larmor frequency dependence on structural anisotropy of magnetically heterogeneous media." Journal of Magnetic Resonance 307 (2019): 106584.
7) Wharton, Samuel, and Richard Bowtell. "Effects of white matter microstructure on phase and susceptibility maps." Magnetic resonance in medicine 73.3 (2015): 1258-1269.
8) Ruh, Alexander, and Valerij G. Kiselev. "Calculation of Larmor precession frequency in magnetically heterogeneous media." Concepts in Magnetic Resonance Part A 47.1 (2018): e21472.
9) Novikov, Dmitry S., et al. "Quantifying brain microstructure with diffusion MRI: Theory and parameter estimation." NMR in Biomedicine 32.4 (2019): e3998.
10) Kiselev, Valerij G., and Dmitry S. Novikov. "Transverse NMR relaxation in biological tissues." NeuroImage 182 (2018): 149-168.
11) Sandgaard, Anders, et al. “Sharper Images in Quantitative Susceptibility Mapping with Magnetometric Dipole Kernels”. ISMRM 2020 #3197.
12) Fisher, Nicholas I., Toby Lewis, and Brian JJ Embleton. Statistical analysis of spherical data. Cambridge university press, 1993.
13) Thorne, Kip S. "Multipole expansions of gravitational radiation." Reviews of Modern Physics 52.2 (1980): 299.
14) Schofield, Marvin A., and Yimei Zhu. "Fast phase unwrapping algorithm for interferometric applications." Optics letters 28.14 (2003): 1194-1196.
15) Zhou, Dong, et al. "Background field removal by solving the Laplacian boundary value problem." NMR in Biomedicine 27.3 (2014): 312-319.
16) Tournier, J-Donald, Fernando Calamante, and Alan Connelly. "Robust determination of the fibre orientation distribution in diffusion MRI: non-negativity constrained super-resolved spherical deconvolution." Neuroimage 35.4 (2007): 1459-1472.
17) Fong, David Chin-Lung, and Michael Saunders. "LSMR: An iterative algorithm for sparse least-squares problems." SIAM Journal on Scientific Computing 33.5 (2011): 2950-2971.
18) van Gelderen, Peter, et al. "A torque balance measurement of anisotropy of the magnetic susceptibility in white matter." Magnetic resonance in medicine 74.5 (2015): 1388-1396.
19) Lee, Hong-Hsi, et al. "Along-axon diameter variation and axonal orientation dispersion revealed with 3D electron microscopy: implications for quantifying brain white matter microstructure with histology and diffusion MRI." Brain Structure and Function 224.4 (2019): 1469-1488.
Figures