3964
Spatial localisation for mapping regional oxygen extraction fraction using parallel transmission saturation pulses at 7 T1Wellcome Centre for Integrative Neuroimaging, FMRIB Division, NDCN, University of Oxford, Oxford, United Kingdom, 2Guy's and St Thomas' NHS Foundation Trust, London, United Kingdom
Synopsis
T2‐relaxation under‐spin‐tagging (TRUST) is a robust spin tagging-based method to quantify oxygen extraction fraction (OEF), but it lacks spatial specificity. Recently, O’Brien et al. proposed a method at 3 T involving multiple saturation pulses to achieve spatial specificity. Parallel transmission (pTx) provides additional degrees of freedom for spatial localisation. A pTx RF pulse design strategy based on a shells trajectory was applied to perform regional OEF measurement at 7 T. Phantom experiments showed that repeating the RF pulses significantly improved the saturation efficiency.
Introduction
Oxygen extraction fraction (OEF) is an important parameter for the understanding of normal and abnormal physiology in the brain. Lu and Ge1 introduced an MR-based method named T2‐relaxation-under‐spin‐tagging (TRUST) to measure OEF by labelling spins in the venous blood and performing pairwise subtraction in the sagittal sinus. OEF is then inferred based on the known relationship between blood T2 and blood oxygenation levels1. Although proven to be a robust non-invasive technique to measure cerebral OEF, TRUST has one key limitation: it does not provide spatial specificity. O’Brien et al. applied a train of water suppression enhanced through T1 effects (WET) saturation pulses to measure regional OEF using selective localised-TRUST (SL-TRUST) at 3 T2. Parallel transmission (pTx) allows different RF waveforms to be played out in different transmit channels, thus providing more degrees of freedom for spatial selectivity to achieve regional saturation. In this work, we present a pTx pulse design strategy to measure regional OEF in the brain at 7 T.Methods
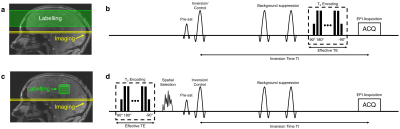
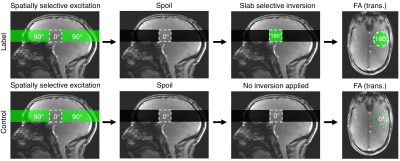
B1+ and B0 maps were acquired from an oil phantom on a Siemens (Erlangen, Germany) Magnetom 7 T scanner equipped with an 8 channel pTx system, and a Nova Medical Inc. (Wilmington, MA, USA) 8Tx/32Rx head coil. B1+ maps were acquired using a 2D "STE first" phase-cycled DREAM sequence3, 4. B0 maps were acquired using a dual-echo gradient-echo sequence.The labelling strategy consists of the following steps. First, a spatially selective pTx pulse excites a 3D shape that excludes the region-of-interest (ROI), which is then saturated by a spoiler gradient. Immediately following, a slab-selective inversion pulse is played out to invert the remaining longitudinal magnetisation in the ROI. In the control case, the same spatially-selective pulse is used, but without a subsequent inversion pulse, thereby creating a signal difference solely from the venous blood in the ROI (Figures 1, 2).
A shells trajectory proposed by Davids et al. was used for the pulse design5. No gradient trajectory optimisation was performed in this study. A cylindrical ROI with a height of 30 mm was chosen. The pulse design employed the variable-exchange algorithm, which was based on a method proposed by Dupas et al.6. The regularisation constant (λ) value was chosen heuristically to produce acceptable results in flip-angle (FA) with a feasible RF voltage. The designed RF pulse was then incorporated into the TRUST sequence to evaluate its performance. T2 encoding, pre-saturation and double inversion pulses were turned off to isolate the effects of the proposed RF pulse. The inversion time was set to the shortest possible value to minimise relaxation effects. Quantitative estimation of saturation efficiency was also performed.
Results
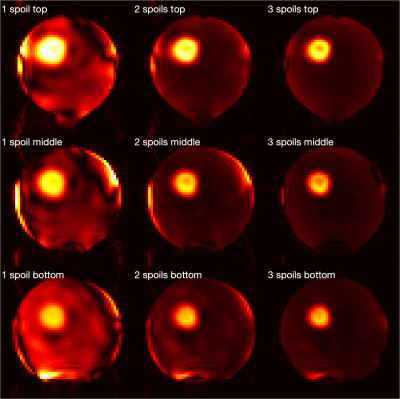
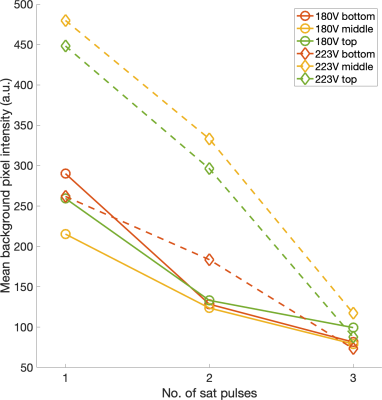
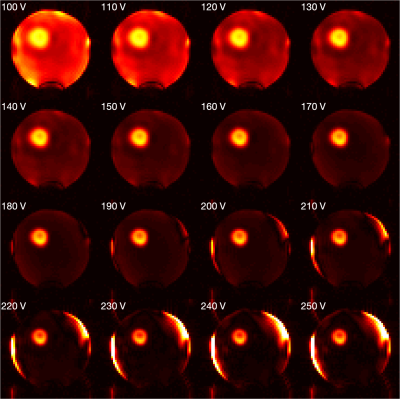
Initial experimental results from one saturation pulse showed that the saturation outside the ROI was not sufficient, and it was hypothesised that the proposed method might benefit from repeating the saturation module. Additional saturation modules further excited and then spoiled the remaining longitudinal magnetisation outside the ROI (Figures 3, 4). The pulse voltage was also altered to investigate its relationship with the saturation efficiency (Figure 5). Although the optimal voltage from simulation was 223V in this case, experimental results showed that a pulse voltage of 180V produced a better saturation. The saturation error was 0.9% for 180V and 2.6% for 223V. However, for these ROI parameters the relative contribution of unwanted signal from regions outside the ROI to the intended signal from within the ROI was estimated to be 21.2% (180V) and 53.7% (223V).Discussion
The main contribution of this study is the addition of a spatially-selective saturation module to the TRUST sequence, thereby allowing the measurement of OEF of a single arbitrary brain region. In addition, the proposed saturation strategy could provide an alternative method for reduced field-of-view imaging. As opposed to directly exciting only the ROI, the proposed strategy can be used to excite and then saturate spins outside the ROI. An image of the localised signal within the ROI can then be acquired using an additional RF pulse. Furthermore, the accuracy of spatial localisation can be enhanced by repeating the proposed saturation module, but not by repeating the inner volume excitation pulses.The high level of contamination, despite low saturation error, is due primarily to the large ratio in volume fraction between the ROI and the regions outside it (6.9% and 93.1% of the volume of the inversion slab, respectively). However, a moderate increase in the ROI volume percentage would substantially decrease the level of signal contamination. The experimental results broadly matched with simulations, but some discrepancies were present. This could be explained by a systematic bias in the B1+ maps that led to under-flipping of the magnetisation. Moreover, the complicated B1+ and B0 patterns at the boundary between the phantom and surrounding air might not have been accurately captured by the B1+ and B0 mapping sequences in this study. Finally, the complex gradient trajectory used in this study might lead to excitation errors due to imperfections of the gradient system.
Conclusion
A pTx RF pulse design is presented for regional OEF measurement at 7 T. Initial phantom results showed the feasibility of the method, but further in vivo studies are needed for the validation of this approach.Acknowledgements
We acknowledge the China Oxford Scholarship Fund, Royal Academy of Engineering, Dunhill Medical Trust, University College Oxford and the NIHR Oxford Biomedical Research Centre. The Wellcome Centre for Integrative Neuroimaging is supported by the Wellcome Trust (203139/Z/16/Z). The support of the UK Medical Research Council UK7T Network is also acknowledged (MR/N008537/1).References
1. Lu, H. and Y. Ge, Quantitative evaluation of oxygenation in venous vessels using T2-Relaxation-Under-Spin-Tagging MRI. Magn Reson Med, 2008. 60(2): p. 357-63.
2. O'Brien, C., et al., Volume-localized measurement of oxygen extraction fraction in the brain using MRI. Magn Reson Med, 2019. 82(4): p. 1412-1423.
3. Nehrke, K. and P. Bornert, DREAM--a novel approach for robust, ultrafast, multislice B(1) mapping. Magn Reson Med, 2012. 68(5): p. 1517-26.
4. Tse, D.H., et al., Encoding methods for B1(+) mapping in parallel transmit systems at ultra high field. J Magn Reson, 2014. 245: p. 125-32.
5. Davids, M., et al., Fast three-dimensional inner volume excitations using parallel transmission and optimized k-space trajectories. Magn Reson Med, 2016. 76(4): p. 1170-82.
6. Dupas, L., et al., Two-spoke placement optimization under explicit specific absorption rate and power constraints in parallel transmission at ultra-high field. J Magn Reson, 2015. 255: p. 59-67.
Figures