3955
Joint optimisation of parallel transmission in 2D spin-echo based sequences
Belinda Ding1, Iulius Dragonu2, Patrick Liebig3, and Christopher T Rodgers1
1Wolfson Brain Imaging Centre, University of Cambridge, Cambridge, United Kingdom, 2Siemens Healthcare Limited, Firmley, United Kingdom, 3Siemens Healthineers, Erlangen, Germany
1Wolfson Brain Imaging Centre, University of Cambridge, Cambridge, United Kingdom, 2Siemens Healthcare Limited, Firmley, United Kingdom, 3Siemens Healthineers, Erlangen, Germany
Synopsis
In this study, we assessed the performance of jointly optimised pTx excitation and refocusing pulses in 2D spin-echo based sequences on a 7T Terra scanner. In conventional pTx acquisitions, the excitation and refocusing pulses are designed independently based a set of fieldmaps. Here, we compared two approaches for jointly optimising the excitation and refocussing against an approach of separately optimised pTx pulses and the traditional circularly polarised pulses. We observed that pTx pulses significantly improve image quality in both phantom and in vivo acquisitions at 7T. The image quality is further improved with joint optimisation of excitation and refocusing pulses.
Introduction
With the recent release of the first clinically approved 7T MRI scanner, we have seen increased interest in utilising ultra-high field scanners. However, one of the main disadvantages of scanning at ultra-high fields is the increase in RF transmit field (B1+) inhomogeneity, resulting in spatially non-uniform signal magnitudes across the brain, including signal dropouts in lower brain regions1. These effects are more severe for higher flip angle pulses such as the refocusing pulse needed in spin-echo imaging. RF parallel transmission (pTx) has been used as one of the techniques to mitigate these effects, but most research have been focused on 3D non-selective kT points pulse designs2. Here, we aim to make an initial comparison in performance between jointly optimised and separately optimised slice-selective pTx spokes pulses in spin-echo based sequences.Pulse Design
Four different groups of pulses were used in both phantom and in vivo acquisitions(Figure 1):- Circularly polarized(CP) pulses: CP pulses were identical to pulses played out on a traditional single transmit system, with all 8 transmit elements playing pulses with identical shapes and amplitudes, varied by a fixed phase shift.
- Separately optimised pTx: Both the excitation pulse and the refocusing pulse were optimised separately, using a linear least square optimisation method3 based on a set of subject-specific B0 and per-channel B1+ fieldmaps.
- Matched excitation pTx: The refocusing pulse was optimised first using the method described for (2). Bloch simulation using Cayley-Klein parameters4, and neglecting intra-pulse relaxation, was then performed to produce a flip-angle map. Based on the FA map, a target excitation pattern was calculated (no longer a homogenous excitation), from which the excitation pulse was then designed with the aim of improving homogeneity in the final refocused signal.
- Jointly optimised pTx: Using pulses designed in (2) as starting points, amplitude and phase weightings for each channel and spoke across both pulses were passed to a simulated annealing global optimisation algorithm. Parameters for simulated annealing include reanneal interval=50; maximum iterations=400; and initial temperature=300. New points for iterations were generated with steps of length square root of temperate, with direction uniformly at random and temperature schedule was InitialTemperature/ln(k), where k is the iteration number until reannealing.
Data acquisition
All experiments were performed on a Magnetom 7 Tesla Terra system (Siemens Healthcare, Erlangen, Germany). Signal acquisition was performed using an 8Tx/32Rx head coil (Nova Medical).Phantom acquisition: A uniform spherical agar phantom was scanned with a spin-echo sequence adapted to include pTx pulses. SE images were obtained with two different sets of FAs (excitation/refocusing FA=60/120 and 90/180) for each group of pulses. For each set of FA and pulse group, 6 TEs ranging from 50ms to 200ms were used. A TR of 3000ms guaranteed more than 90% recovery of longitudinal magnetisation. Phantom experiments had an isotropic resolution of 3mm. T2 maps were computed offline by fitting to a mono-exponential decay.
In vivo acquisition: A healthy volunteer (male, age = 27 years) was scanned using a 2D SE echo-planner imaging(EPI) sequence adapted to include pTx pulses. Single slice SE-EPI images were obtained with two different sets of FAs (excitation/refocussing FA=70/140° and 90/180°) for each group of pulses. Other imaging parameters included: FOV=240×240 mm2; voxel size=0.5×0.5×3(slice)mm3; GRAPPA acceleration factor R=3 and 6/8 partial Fourier; TE=60 ms; and TR=6000 ms.
Results and discussion
SE images acquired in phantom and the resultant T2 maps are shown in Figure 2. The distributions of signal intensity and fitted T2 across the phantom are shown in Figure 3. Compared to CP pulses, all three pTx optimisation methods gave narrower (better) distributions of signal intensity and fitted T2 values.In vivo SE-EPI images are shown in Figure 4 and 5. For the case of CP pulses, clear signal dropouts can be observed in the temporal lobes. All three proposed pTx pulse optimisation methods are able to recover the signal in this area. Similar image quality with excellent homogeneity is obtained for all three pTx pulse optimization methods. Subtle differences can be detected on closer inspection as displayed by arrows in Figure 5. Joint optimisation of both pulses (method 4) outperformed the other two methods by producing clearer definition in both the pons (yellow boxes in Figure 5) and tip of the temporal pole (red arrows in Figure 5).
Conclusion
In this abstract, we have shown the importance of using parallel transmit pulses when acquiring SE based data at 7T. Joint optimisation of excitation and refocusing pulses improves the image quality. We believe that these results will be applicable to a wide range of SE-based sequences which requires slice selective excitation such as diffusion imaging and inner-volume selected SE-MRSI. Further optimisation of the joint pulse design pipeline including volume-by-volume optimization for whole brain imaging will allow for very homogeneous resulting magnetisation and increased performance compared to separately optimised pTx pulses.Acknowledgements
BD is supported by Gates Cambridge Trust. CTR is funded by a Sir Henry Dale Fellowship from the Wellcome Trust and the Royal Society [098436/Z/12/B]. This study was funded by the NIHR Cambridge Biomedical Research Centre and MRC Clinical Research Infrastructure Award for 7T and has also received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement No 801075.References
- Wu X, Auerbach EJ, Vu AT, et al. High-resolution whole-brain diffusion MRI at 7T using radiofrequency parallel transmission. Magn Reson Med. 2018;80(5):1857-1870. doi:10.1002/mrm.27189
- Gras V, Mauconduit F, Vignaud A, et al. Design of universal parallel-transmit refocusing k T -point pulses and application to 3D T 2 -weighted imaging at 7T. Magn Reson Med. 2018;80(1):53-65. doi:10.1002/mrm.27001
- Setsompop K, Wald LL, Alagappan V, Gagoski BA, Adalsteinsson E. Magnitude least squares optimization for parallel radio frequency excitation design demonstrated at 7 Tesla with eight channels. Magn Reson Med. 2008;59(4):908-915. doi:10.1002/mrm.21513
- Pauly J, Nishimura D, Macovski A, Roux P Le. Parameter Relations for the Shinnar-Le Roux Selective Excitation Pulse Design Algorithm. IEEE Trans Med Imaging. 1991;10(1):53-65. doi:10.1109/42.75611
Figures
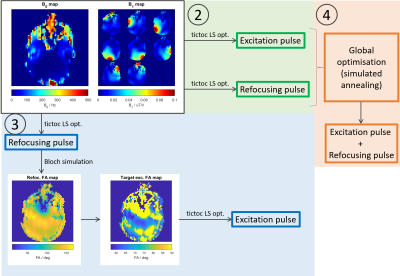
Figure 1: Pulse optimisation work flow for (2)
separately optimised pTx pulses, (3) matched excitation pTx pulses and (4)
jointly optimised pTx pulses.
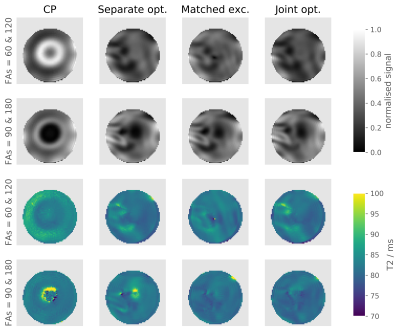
Figure 2: Top two rows: Normalised SE image
acquired in a spherical agar phantom with a TE of 100 ms. Two different sets of
FAs were used: excitation/refocusing FA =
60 /120 (first row) and 90/180 (second row). Bottom two rows: Calculated
T2 maps in phantoms using SE images acquired with 6 different TEs
and a mono-exponential decay signal fitting.
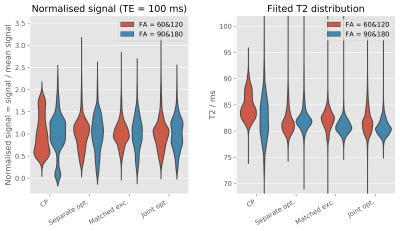
Figure 3: Left: Distribution of signal intensity
(normalised to the mean signal intensity) in a spherical agar phantom with a TE
of 100 ms. Right: Distribution of fitted T2 in the same phantom for
different pulses and different flip angles.
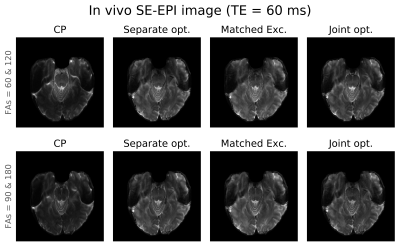
Figure 4: Representative SE-EPI images acquired in
a healthy volunteer with all 4 proposed methods of pulse design of pulses and
two different values for excitation/refocusing = 60°/120° (top row) and
90°/180° (bottom).
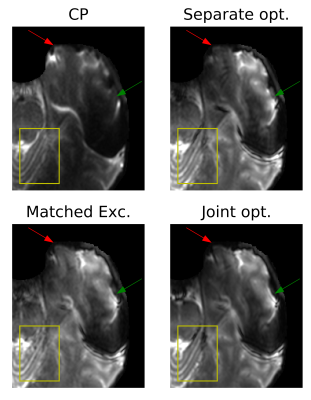
Figure 5: Zoomed in SE-EPI images acquired with
excitation FA of 60° and refocusing FA of 120°. Significant signal dropouts can
be observed in the case of CP pulses (green arrows). Excellent signal
homogeneity is observed when using any of the 3 pTx optimisation methods.
Signal losses at the edges (red arrows) are further recovered with matched
excitation and joint optimised pulses.